This week's life sciences news includes an advancement in cancer detection, a new chairman for a Philadelphia cell therapy company, a potential new market for a pneumonia drug, and more.
Here's the roundup:
Penn Medicine
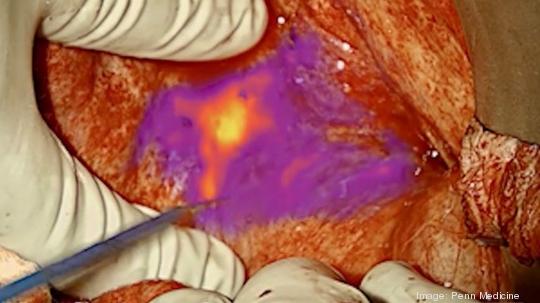
The FDA approved an ovarian cancer imaging drug, known as Cytalux, that uses an approach pioneered by Penn Medicine surgeons.
Cytalux, which will be marketed by On Target Laboratories of Indiana, is attracted to ovarian cancer tissue and illuminates it when exposed to fluorescent light, allowing surgeons to more easily find and more precisely remove the cancer.
Physicians at the Center for Precision Surgery in the Abramson Cancer Center at the University of Pennsylvania led one of the largest clinical trial site in the country for the drug.

“Lighting up cancer, which helps to identify lesions that may be difficult to find — especially in the presence of scar tissue or other organ damage — enables the more complete identification and surgical removal of cancer that could have otherwise been missed,” said Dr. Janos L. Tanyi, an associate professor of obstetrics and gynecology in the Perelman School who was principal investigator for Penn’s clinical trials of the imaging agent.
In another development this week related to the same cancer, Scientists at the Wistar institute, led by Rugang Zhang, have identified two genes that play a critical role in protecting ovarian cancer from the immune system.

Their findings were published in the American Association for Cancer Research journal Cancer Immunology Research. Wistar researchers believe the discovery could help with the development of new treatments in which ovarian cancer, and other types of cancer, are more vulnerable to immunotherapy.
Carisma Therapeutics
The Philadelphia cell therapy company named Sanford Zweifach as its board chairman.
Zweifach has three decades of experience in the life sciences industry. He was most recently chairman of Palladio Biosciences and Janpix, which were merged into Centessa Pharmaceuticals (NASDAQ: CNTA). He is also executive chairman of Kaerus Biosciences, a French biotech company, and an advisor to several life science companies across several therapeutic areas. Zweifach's resume also includes serving as a venture partner at Medicxi, co-founder and CEO of Nuvelution Pharma, co-founder and CEO of Ascendancy Healthcare, and partner at Reedland Capital Partners.

“Sandy’s extensive experience across the life sciences industry is invaluable as a counselor to, and partner, in Carisma’s continued growth,” said Steve Kelly, the company's CEO.
Nabriva Therapeutics
A new drug application for lefamulin filed by the Fort Washington biopharmaceutical company's partner, Sumitomo Pharmaceuticals, was accepted for review by the Chinese Center for Drug Evaluation.
Lefamulin, marketed in the United States by Nabriva as Xenleta, is used to treat community-acquired pneumonia in adults. The drug is available in oral and intravenous formulations.
Nabriva (NASDAQ: NBRV) entered into an agreement in May 2021 giving Sumitomo development and commercialization rights for lefamulin in the greater China region. The application is seeking approval for the drug in Mainland China. Sumitomo received approval for lefamulin in Taiwan in September.
Ted Schroeder, CEO of Nabriva, said the two companies are working collaboratively throughout the health authority review process to get the drug approved in Mainland China.
Quick hits
Adare Pharma Solutions, a North Jersey contract development and manufacturing organization (CDMO), has acquired Frontida BioPharma, a Philadelphia CDMO. Financial terms of the deal are being kept confidential. …Plymouth Meeting-based biotechnology company Inovio (NASDAQ: INO) said it is "rapidly moving" to evaluate its Covid-19 DNA vaccine candidates INO-4800 and INO-4802 against the emerging Omicron variant. Inovio said it has also initiated pre-clinical development of an Omicron-specific DNA vaccine candidate.