This week's life sciences industry news includes an encouraging development for a Philadelphia immunotherapy company's lead drug candidate, an exclusive partnership for a Malvern medical device company, a startup's debt financial deal and more.
Here's the roundup:
Imvax
The Philadelphia clinical-stage biotechnology company, which is developing personalized and whole tumor-derived immunotherapies, presented data at a national conference that shows the mechanisms by which its lead new drug candidate, IGV-001, produces broad immune activation.
Study results Imvax presented at the Society for Immunotherapy of Cancer's annual meeting highlighted the effects of IGV-001 — which the company is developing initially to treat glioblastoma, a type of brain cancer — on inducing both innate and adaptive immune responses to tumor cells.
John P. Furey, CEO of Imvax, said the data substantiate the anti-tumor effects the company observed in prior clinical trials of IGV-001, and the insights from the study will aid its ongoing clinical development efforts for IGV-001 in glioblastoma.
“Importantly," Furey said, "these studies also underscore the potential expansion of Imvax’s approach to a wide range of solid tumors and bolster our ongoing preclinical work in hepatocellular, ovarian, pancreatic, and other cancers.”
Telesis Therapeutics
The Philadelphia biopharmaceutical company raised $615,000 in a debt financing, according to documents filed with the Securities and Exchange Commission.
Telesis, led by founder and CEO Maurice Hampton, is developing a potential treatment for pancreatic cancer.

The company's lead new drug candidate, TTL-315, was discovered by a former researcher at the Lankenau Institute for Medical Research in Wynnewood. In preclinical testing, the small molecule demonstrated effectiveness in treating solid tumors associated with breast, lung and skin cancers.
Last year, Telesis signed a collaborative research and development agreement with the National Cancer Institute's Frederick National Laboratory for Cancer Research in Maryland.
Neuronetics
The Malvern-based medical device company entered into a partnership with River Region Psychiatry Associates, a leading provider of mental health services in Alabama, Georgia, Kentucky and Tennessee.
Under the agreement, Neuronetics (NASDAQ: STIM) will be the exclusive supplier of transcranial magnetic stimulation equipment to River Region and its clinics.
Neuronetics is the developer of the NeuroStar Advanced Therapy system that uses transcranial magnetic stimulation to target key areas of the brain that are under-active in people with major depressive disorder. The system has been used for more than 4 million treatments delivered to more than 110,000 patients.
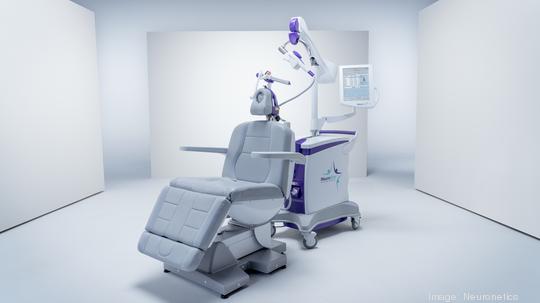
The first NeuroStar Advanced Therapy system is expected to be available to patients in the Pelham/Helena community in Alabama.
“As depression rates increase, this partnership will provide more patients access to a non-drug treatment option for depression,” said Keith J. Sullivan, CEO of Neuronetics.
Quick hits
Philadelphia cell therapy developer Cabaletta Bio (NASDAQ: CABA) has promoted two people to senior leadership positions. Dr. Samik Basu, previously vice president of preclinical research and translational medicine, is now chief scientific officer. Heather Harte-Hall, previously vice president of quality and compliance, is now chief compliance officer. … Fort Washington-based Nabriva Therapeutics (NASDAQ: NBRV) said the oral formulation of Xenleta, the company's community-acquired bacterial pneumonia drug, is now available in a 10-count oral pack in the United States through major specialty distributors. Ted Schroeder, CEO of Nabriva, said the new package size offers pharmacies an option to "expand product availability in a cost-effective way to service their local patients and physicians by providing patients a convenient package that contains a complete 5-day course of oral treatment."