This week's life sciences industry roundup includes one Philadelphia-area company securing Food and Drug Administration marketing approval for a medical device and another submitting an application with the FDA to conduct late-stage testing of a Covid-19 vaccine.
Here's the roundup:
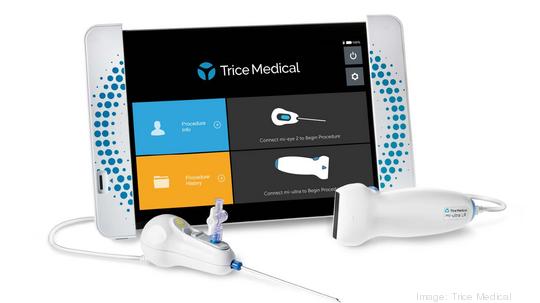
Trice Medical
The Malvern medical device developer received FDA marketing clearance for the company's 25-degree mi-eye 3 Needlescope — a single-use, single-hand device used in arthroscopic and endoscopic procedures.
Trice CEO Mark Foster said the instrument allows physicians to expand the field of view and access areas of joints "as no arthroscope has done before.”
The company said its mi-eye 3 Needlescope camera's streamlined design eliminates undesirable aspects of existing reusable arthroscopes, including the two-handed light source and angle rotation requirements and multiple accident-prone cables. It also removes costly equipment, sterile processing, and other expenses.
Ocugen
The Malvern biopharmaceutical company submitted an investigational new drug application with the FDA seeking approval to conduct a Phase 3 clinical trial to evaluate the effectiveness of the Covid-19 vaccine Covaxin, which is already approved for use in India.
Malvern-based Ocugen (NASDAQ: OCGN) in February formed a partnership with India-based Bharat Biotech, the developer of Covaxin, to bring the vaccine to the U.S.
“We are very excited to take this next step in the development of Covaxin, which we hope will bring us closer to introducing a different type of Covid-19 vaccine to the American public,” said Dr. Shankar Musunuri, CEO and co-founder of Ocugen. “We are hopeful that the study conducted under the [investigational new drug application], if allowed to proceed, will help demonstrate that the data from India will be applicable to the U.S. population.”

Covaxin, Ocugen said, is a highly purified and inactivated vaccine that is manufactured using the same vero cell manufacturing platform that has been used in the production of polio vaccines for decades.
Windtree Therapeutics
The Warrington-based biotechnology and medical device company was issued a notice of allowance by United States Patent and Trademark Office for patents related to the administration of its experimental heart drug istaroxime.
Windtree (NASDAQ: WINT) is conducting mid-stage clinical testing of istaroxime to determine its effectiveness as an acute heart failure treatment.
A notice of allowance is issued by the patent office to indicate that the application has passed examination. When the patent is issued to Windtree in the near future, it will provide new intellectual property protection for istaroxime until late 2039.

Windtree CEO Craig Fraser said the company plans to start its next study of istaroxime in acute heart failure by mid-2022.
Quick hits
Blue Bell-based Inovio (NASDAQ: INO) completed its enrollment of 220 participants for a Phase 1 study of its DNA vaccine candidate INO-450 for Lassa fever. The study is ongoing at the Noguchi Memorial Institute for Medical Research in Accra, Ghana, and is the first vaccine clinical trial for Lassa fever conducted in West Africa, where the viral illness is endemic. … Philadelphia immunotherpy developer Context Therapeutics (NASDAQ: CNTX) and the Wisconsin Oncology Network said the first patient has been dosed in a Phase 2 trial of OnapriStone, Context's experimental treatment used as part of a combination therapy for women with metastatic breast cancer. The trial will enroll up to 39 patients. … OptiNose (NASDAQ: OPTN) completed patient recruitment for the second of two Phase 3 clinical studies evaluating the safety and effectiveness of Xhance nasal spray as a treatment for patients with chronic sinusitis, a disease that causes inflammation of the mucous membranes that line the sinuses in the nose. The Yardley biopharmaceutical company expects results from the first trial in the first quarter of 2022 and from the second trial in the second quarter of 2022. Xhance, which has FDA approval to treat nasal polyps, is a drug-device combination product that uses an Optinose Exhalation Delivery System to deliver medication to high and deep regions of the nasal cavity.







