Sklip Inc., a Lake Oswego-based telehealth company focused on dermatology, received a Breakthrough Device Designation from the U.S. Food and Drug Administration for its artificial intelligence system, the company announced.
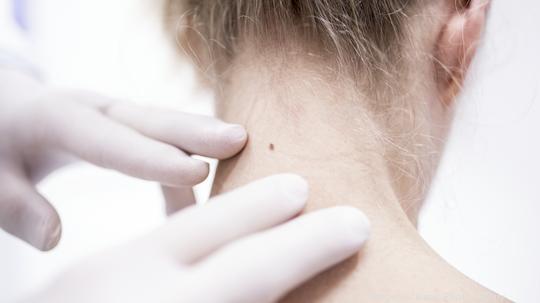
The Sklip System AI uses medical-grade digital dermoscopy images to help in the early detection of skin cancer. When detected early, the five-year survival rate for melanoma is 99%, according to Sklip.
The Sklip Dermatoscope, listed on Sklip’s site for $149.99, can be clipped onto a phone or tablet without an adapter. When aligned with the phone’s camera, it takes high-definition images to store for comparison or share with a doctor for mole analysis.
Breakthrough Device Designation expedites the development and review of products to provide quicker access to medical devices for life-threatening or debilitating diseases and conditions.
The company was founded by two practicing dermatologists, Dr. Alexander Witkowski and Dr. Joanna Ludzik, who co-direct the Skin Imaging and Technology Center at Oregon Health & Science University.
Sklip’s announcement says it is “collaborating with multiple academic centers through active clinical trials in the United States.”





