News out of Greater Philadelphia's life sciences industry this week includes a stock buyback plan, progress being made on a potential chronic sinusitis treatment, a biotech firm's partnership for health equity, and more.
Here's the roundup:
Globus Medical
The medical device developer's board of directors voted to expand the Audubon company's share repurchase program.
The board authorized the repurchase of an additional $200 million of Globus common stock. The expansion, when combined with the $95.3 million currently available under the company's existing share repurchase program, approved in March 2020, provides Globus with more than $295 million available for future share repurchases.
“Our announcement reflects the continued confidence that we have in our long-term growth profile and underlying earnings strength,” said David Paul, executive chairman of Globus, in a statement. “We continue to view Globus as an attractive investment opportunity over the long-term, and our strong balance sheet provides us with the financial flexibility to drive continued strategic investments — both organic and inorganic — while also providing returns to our shareholders."
The specific timing and actual number of shares repurchased will be determined by factors such as market price, general business and market conditions, applicable legal requirements, and alternative investment opportunities. Globus intends to use its cash reserves to fund the share repurchase program.
Globus Medical (NYSE: GMED), founded in 2003, specializes in products that enable surgeons to promote healing in patients with musculoskeletal disorders.
The company generated net sales of $958.1 million last year, an increase of 21.4% compared to 2020. Its net income was $149.2 million, a decrease of 71.5% from the prior year. The drop, according to the company, was driven primarily by a fourth quarter deal in which Globus acquired substantially all the assets of Capstone Surgical Technologies, which develops advanced drill and robotic surgery platforms. The acquisition included a $24.5 million cash payment at closing.
Optinose
The Yardley pharmaceutical company announced positive results from late-stage testing of Xhance as a treatment for chronic sinusitis, an inflammatory disease that afflicts 30 million adults in the United States.
The 332-patient study is one of two Phase 3 clinical trials Optinose (NASDAQ: OPTN) is conducting. Results from the second study are expected in the second quarter of this year.
Dr. Ramy Mahmoud, the company's president, said the study results showed improvement in symptoms, which include nasal congestion and facial pain or pressure, and in the amount of inflammation in the sinuses.
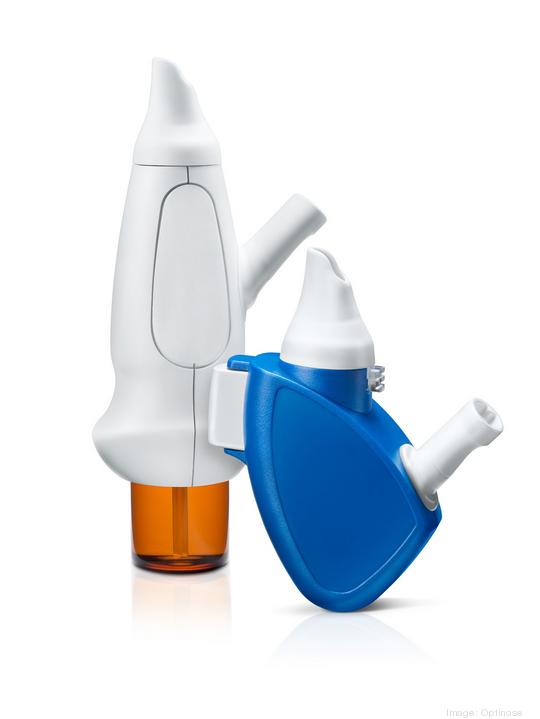
Xhance is a drug-device combination product that uses the exhalation delivery to get a topical anti-inflammatory corticosteroid to high and deep regions of the nasal cavity. Xhance was approved by the Food and Drug Administration in 2017 as a treatment of nasal polyps in patients 18 years of age or older.
Integral Molecular
The Philadelphia biotechnology company has formed a partnership with Philadelphia Fight to provide financial and in-kind donations, as well as volunteer efforts, to support health care access and equity in the city.
"Integral Molecular has deep ties to Philadelphia, and for the last 20 years we have been proud to give back through local educational program," said Sharon Willis, co-founder of Integral Molecular, noting the company's participation with the University City Science Center's BULB lab program, the FirstHand STEM program, and the Wistar Institute's Biomedical Technician Training Program. "We are excited to support Fight's vital mission and continue our commitment to the community."
Philadelphia Fight operates federally qualified community health centers that provide culturally competent and comprehensive health services to all patients regardless of their income, insurance, or immigration status.
Integral Molecular develops and applies innovative technologies — with a focus on membrane proteins and antibodies — for difficult-to-treat diseases, and, more recently, to apply their technologies to combat Covid-19.
WuXi Advanced Therapies
The Philadelphia-based wholly owned subsidiary of WuXi AppTec this week launched a new technology designed to improve the process for making cell and gene therapies.
The technology — called tetracycline-enabled self-silencing adenovirus, or TESSA — represents a novel process for the "transfection-free" scalable manufacturing of adeno-associated viruses (AAV) used in the delivery of call and gene therapies. According to WuXi, TESSA will expedite AAV manufacturing and significantly reduce the cost for manufacturing such treatments.

"The TESSA system is a game changer for the manufacture of AAV and the advancement of cell and gene therapies to benefit patients." said Dr. Ryan Cawood, chief scientific officer of WuXi Advanced Therapies, a contract testing development and manufacturing organization.
Cawood said by improving scalability, reducing process complexity and lowering the costs of gene therapy manufacturing, TESSA will better enable WuXi's global partners to "develop and deliver life-saving gene therapies faster to more patients in need."
Quick Hits
Dr. Jane Huang is joining Prelude Therapeutics (NASDAQ: PRLD), taking the newly created position of president and chief medical officer at the Wilmington-based precision oncology company. Huang joins Prelude from BeiGene, where she is chief medical officer for hematology. … Passage Bio (NASDAQ: PASG), has dosed its first patient in its global Phase 1/2 clinical trial testing its experimental gene therapy PBKRO3 as a treatment for early infantile Krabbe disease, a neurological disorder. … Philadelphia medical technology company Strados Labs was awarded a two-year, $990,118 Small Business Innovation Research Phase II grant from the National Science Foundation. The funds will be used by the company to advance its smart sensors designed to provide early detection and predictions of infectious respiratory diseases. … Malvern-based Baudax Bio (NASDAQ: BXRX) regained compliance with Nasdaq's minimum bid price requirement of $1 per share. Nasdaq staff made the determination of compliance after the company's bid price closed above $1 per share for 10 consecutive business days from Feb. 16 to March 2. Baudax markets Anjeso, an intravenous, non-steroidal anti-inflammatory for the management of moderate to severe pain.