This week's update on Greater Philadelphia's life sciences industry includes a translational research services agreement, Covid-19 vaccine news, a new product approval, a product launch, a CEO change, and more.
Here's the roundup:
Verismo Therapeutics
The Philadelphia cell therapy company spun out of the University of Pennsylvania two years ago signed a translational research services agreement with Penn that includes a manufacturing partnership with organizations within the university's Perelman School of Medicine.
The entities included in the agreement include a vector manufacturing facility at the medical school's Center for Advanced Retinal & Ocular Therapeutics.
Financial terms of the deal were not disclosed.
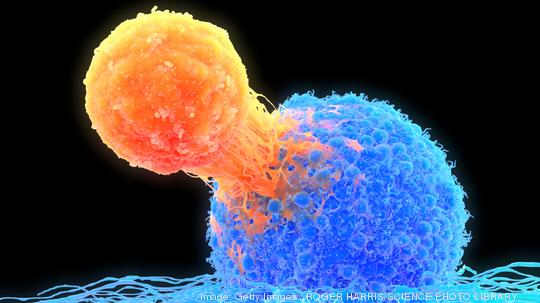
The agreement will support the clinical development of Verismo's first new drug candidate, SynKIR-100. The experimental cell therapy is being developed to treat late-stage ovarian cancer and mesothelioma, a type of cancer tied to asbestos exposure.
Verismo describes itself as a "pioneer in multi-chain CAR-T technology."
In therapy using CAR-T cells, an acronym for chimeric antigen receptor, a patient's T cells are removed and genetically modified in the laboratory. After they are re-injected into a patient they are better able to attack cancer cells. T cells are a type of immune system cell. Verismo said its founders — Dr. Bryan Kim, Dr. Michael Milone and Dr. Donald Siegel — re-invented the technology to create what it calls KIR-CAR and its SynKIR platform to potentially treat solid tumors. KIR-CAR, according to company, is being designed to provide a natural on-and-off stimulation to T cells, without triggering T-cell exhaustion.
Dr. Bryan Kim, CEO of Verismo, said the expertise of Penn's manufacturing teams will allow Verismo to more effectively initiate first-in-human clinical testing of its SynKIR products.
The company anticipates initiating human studies of SynKIR-110 in the first quarter of 2023.
Ocugen
The Malvern biopharmaceutical company, which is attempting to bring a Covid-10 vaccine approved in India to the United States and Canada, commissioned a survey to gauge attitudes about Covid vaccines.
The Harris Poll survey of more than 2,000 adults showed a majority of Americans want more Covid-19 vaccine options from which to choose. The survey found 73% of the respondents would like to see additional vaccine options available that are developed from a more traditional method, such as those used for diphtheria, mumps, chickenpox, or polio.

The survey showed that 38% of parents with children ages 5 and under said they "definitely/probably" will not get their children vaccinated for Covid-19 with the existing vaccines if and when they are eligible.
Ocugen (NASDAQ: OCGN) last year entered into a partnership with Bharat Biotech to bring Bharat's Covid-19 vaccine, called Covaxin, to this country. Covaxin is described as a highly purified and inactivated vaccine that is manufactured using a vero cell manufacturing platform. The platform has been used in the production of the inactivated polio vaccine for the past 35 years, as well as other traditional childhood vaccines.
Dr. Shankar Musunuri, Ocugen's co-founder and CEO, said the survey results show most parents have concerns about giving their children the Covid-19 vaccines now in the marketplace that use messenger-RNA technology to teach cells how to make a protein that will trigger an immune response against the virus.
“All safe and effective options should be considered to manage the pandemic," Musunuri said.
Ocugen in November submitted an emergency use authorization application to the Food and Drug Administration for use of Covaxin in children ages 2 to 18.
Amring Pharmaceuticals
The Berwyn-based generic and brand pharmaceutical company received FDA approval for lamotrigine.
Lamotrigine is the generic equivalent of Lamictal, a GlaxoSmithKline drug approved as a treatment for epilepsy and bipolar disorder. The drug was first approved by the FDA in 1994.
Amring's lamotrigine product is available in 25-,50-, 100- and 200-milligram orally disintegrating tablets.
It will be available as an adjunctive therapy in epilepsy patients age 2 and older, as a monotherapy in epilepsy patients age 16 and older, and as a maintenance therapy for patients with bipolar I disorder who are being treated for acute mood episodes with standard therapy.
Amring is led by CEO Daniel Carbery. The company is a subsidiary of Sever Life Sciences, a global private specialty pharmaceutical company based in the Netherlands.
NexGel
The Langhorne medical device company launched its latest product: the Medagel bug bite relief patch.
The cooling patches use NexGel’s hydrogel technology to provide instant relief to irritated skin caused by insect bites. The patches, which are chemical- and latex-free, are infused with skin-calming Arnica flower and pain-reducing Lidocaine to help reduce discomfort.

“When an insect bites you – be it a mosquito, wasp, and the like – your body creates a natural immune response which typically results in swelling of the skin and an insatiable itch, both leading to discomfort,” said Adam Levy, CEO of NexGel (NASDAQ: NXGL), in a statement. “That intense itchy sensation creates an overwhelming urge to scratch the skin until it’s raw."
The Medagel bug bite relief patches, he said, are designed to help users maintain healthy skin barriers by reducing inflammation and the desire to scratch.
Quick hits
Avalo Therapeutics (NASDAQ: AVTX), a biopharmaceutical company with operations in Rockville, Maryland, and Wayne, announced a leadership transition. Mike Cola, the company's CEO, and Schond Greenway, its CFO, have left the company to pursue other interests. Garry Neil, previously the company's chief scientific officer, has taken over as CEO. Chris Sullivan was promoted from chief accounting officer to CFO. ... Malvern-based Baudax Bio (NASDAQ: BXBX) enacted a one-for-35 reverse stock split, which became effective Feb. 16. The biopharmaceutical company, which markets the pain medicine Anjeso, made the move to get its stock into compliance with Nasdaq's $1 minimum bid listing requirement. ... SwanBio Therapeutics, a Philadelphia gene therapy company, was granted Fast Track designation for its lead drug candidate, SBT101, from the FDA. The designation is designed to facilitate the development, and expedite the review, of experimental drugs targeting serious conditions with unmet medical needs. SBT101 is under development for the treatment of adrenomyeloneuropathy, a degenerative spinal cord disease. SwanBio plans to initiate a Phase 1/2 clinical trial for the gene therapy candidate during second half of this year.