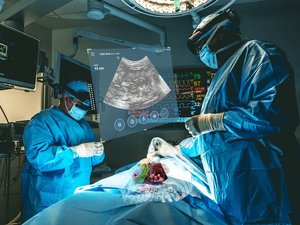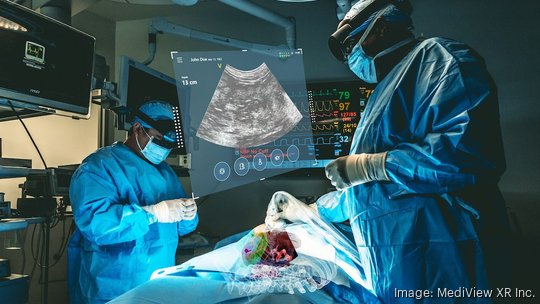
Cleveland medical technology startup MediView XR Inc. has won U.S. Food and Drug Administration clearance to market its platform that uses augmented reality to give surgeons a kind of "X-ray vision" to navigate the body during minimally invasive procedures.
The platform, known as XR90, is intended to be used along with ultrasound and CT-guided, needle-based procedures for soft tissue and bone.
Thursday's announcement marked MediView's first 510(k) clearance, but it also was the first-ever clearance for an augmented reality device that uses live imaging and 3D visualization for pre- and intra-operative procedures, said Adam Cargill, MediView's director of quality, regulatory and clinical affairs, in a statement.
The clearance "sets the stage for further advancements in augmented reality in the health care space," Cargill said.
"We are in a new era of visualization, collaboration and data insights in health care that can create patient, clinician and financial benefits," said Mina Fahim, president and CEO of MediView, in the statement.
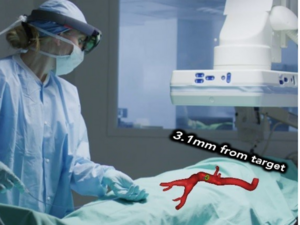
XR90 uses Microsoft's HoloLens 2 AR headset to provide physicians with 3D "X-ray vision" during procedures — giving them the ability to visualize a patient's internal anatomy under their skin, including bone, tissue, organs and blood vessels, MediView said.
MediView has raised about $29.5 million in funding to date, including $15 million in May and $10 million in March 2022.
The company that refined and expanded upon intellectual property originating at Cleveland Clinic also struck an agreement to advance its technology with clinical, technology and research collaboration from Mayo Clinic.
In addition, MediView is collaborating with GE HealthCare to integrate medical imaging with mixed-reality solutions for the interventional health care space, the company said.