The first time Lisa Anderson received a human pancreas, she was shocked.
The organ had arrived in a series of sterile plastic bags filled with solution, then placed into a styrofoam cooler cushioned by crushed ice to keep it cold. It was as if it were prepared for a picnic, not for transplantation into a human body in need.
Anderson, at the time a junior faculty member at Harvard Medical School, thought there might have been a mistake. She speculated that the agency that had sent the pancreas might have packaged it so rudimentarily because she had ordered it for an academic laboratory, not a hospital. She called the agency.
“Could you do me a favor and and package the pancreas as if it were for human transplantation in a clinical setting?” Anderson recalled saying. “And there was a long pause on the phone. Then, somebody said, ‘Well, this is how we do it.’ I said, ‘What, just for the pancreas?’ They said, ‘No, this is actually how organs are transported for transplantation.’”
Anderson couldn’t believe it: “I thought it was lacking the necessary respect,” she said. Plus, on a scientific level, the crude packaging method actually skewed the results of her experiment. Now, her lab was working with imperfect data.
This is a problem doctors experience every day. Often, human organs intended for transplant are rendered nonviable because of the way they are transported. If kept too cold by the crushed ice, surface tissue can freeze. This is especially true of pediatric organs—too small to resist the encroaching ice crystals that get inside and disrupt living cells.
Anderson set out to develop a new system for organ transportation.
With connections from both the venture capital and the academic world—Anderson was previously a vice president at Seed One Ventures and has held academic positions at the University of Cambridge, the Broad Institute and Brigham and Women’s Hospital along with Harvard Medical School—Anderson worked to create a new kind of organ delivery device, one that would ensure human organs both received the necessary respect from doctors and be viable upon arrival in a hospital room. She teamed up with medical device veteran Bill Edelman, who has nearly 35 years of experience in this field.
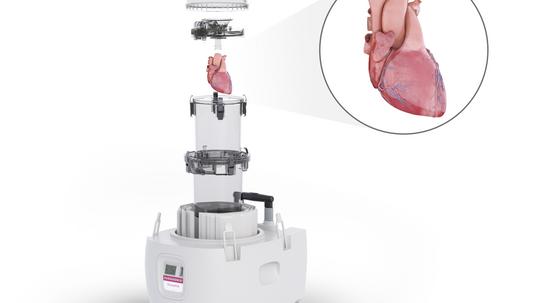
Now, Anderson is president and COO of Paragonix, a nearly-10-year-old startup based in Cambridge. Edelman is CEO and chairman of the board. The company’s flagship product is a device called SherpaPak. It’s a far cry from the styrofoam container in which Anderson received the pancreas back at Harvard.
“It basically consists of metal canister, in which the heart is suspended, so it's not in physical contact with anything,” Edelman said. “Once the heart is inside the canister, it's flooded with preservation fluid, which is already in use by surgical teams. When it's loaded into our Sherpa system, the properties of Sherpa maintain the temperature between 4 degrees Celsius and 8 degrees Celsius throughout the entirety of the transport time. That enables effective suspension of the metabolism of the heart without inducing freezing and avoiding that potential for damage.”
SherpaPak has been approved for use in the U.S. by the Food and Drug Administration (FDA). It has also received CE registration in Europe, a certification mark that indicates conformity with health, safety and environmental protection standards for products sold within the European Economic Area.
Paragonix has two streams of revenue: the first from direct sales of SherpaPak and the second from outside investment. Edelman could not disclose any specifics when it came to private investment, but he said Paragonix has a "large group" of private investors who have also put funding into other companies nationwide.
The company's primary focus for the moment is on hearts, but it has also begun to expand into transportation of kidneys and lungs. Hearts, Edelman said, were the company’s initial focus because the need for better cardiac transport is so immediate.
Some 30 hospitals have been using Paragonix’s SherPak system. So far, Paragonix has experienced an incredible success rate: 100 percent. And nearly one year ago today, Paragonix earned 510(k) clearance from the FDA to transport pediatric hearts using SherpaPak.
For Anderson, that is the most profound experience: to have created a medical device that can save the life of a child, especially given that one life saved—when it comes to organ transplantation—means that another child, and another family, has suddenly lost so much.
“I’m a mother of two young children, and the use of the device for pediatric heart recovery has been one of the most gratifying experiences for me,” Anderson said. “To be involved in a case where you can ensure that a child’s heart is treated respectfully and preserved optimally so another child can regain life, that is one of the most gratifying experiences for myself and I think for all of Paragonix’s employees and team members.”








