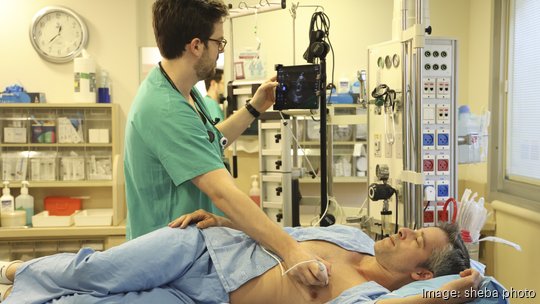
An Israel-based medical technology company has moved to Boston after getting FDA approval for a device that helps diagnose heart disease quickly.
Aisap received 510(k) clearance from the he FDA for a new medical device that helps doctors perform cardiac ultrasounds at the patient's bedside in less time than a traditional echocardiogram.
Former CEO Roni Attali moved to Boston earlier this week after changing positions to become the latest U.S. general manager and chief sales officer on Aug. 1.
The point-of-care assisted diagnosis — or POCAD, as Attali called it — is powered by AI. It has 12 different algorithms that combine four computer-assisted diagnosis modules of valvular pathologies and eight key measurements into a single cardiac ultrasound software package.
The system was born out of the fact that there were not enough echocardiograms to go around at the Aisap's hospital in Israel, a problem shared by the US healthcare system, according to Attali.
Because so-called "echos" take so long, and require a specialist to read them, backlogs are a frequent problem. With the POCAD system, doctors can take visual readings faster, and the AI integration helps them interpret the results.
According to Attali, while a traditional echocardiogram takes 45 minutes, the equivalent exam with the POCAD system only takes five. The new system helps reduce the wait time for echos and helps physicians catch potential risks much earlier.
“At the end of the day, we're not reducing the amount of echos. The echo is always going to be in a full capacity,” said Attali. “What we're changing is the triage to the echo. What we're changing is that the person getting the echo is a person that needs an echo, which will probably turn into a procedure in the hospital.”
Attali says the Aisap system’s AI was trained on hundreds of thousands of studies comprising over 24 million echo video clips. Mass General Brigham was one of the hospitals that participated in the system's testing. The Boston Hospital found that Aisap's device measures were close to the mean of three MGB cardiologists, with a 93% sensitivity and 93% specificity.
So far, Attali is the first part of Aisap to come to the US and start its market expansion. According to Attali, the company plans to have an office building in Boston within six months.
Attali wants to fill three to four roles and build the marketing and sales team.
“The crazy thing about our solution is that it's not just for the hospital setting, right? It's for rural care, for primary care, for the army, and it could be for sports teams, right? Because, again, it's a handheld device. So the potential is huge,” said Attali