Rockville’s ReveraGen BioPharma Inc., a 15-year-old Children’s National Hospital spinout, is preparing for the launch of its neuromuscular disease drug — and sees an opportunity to advance it for other conditions.
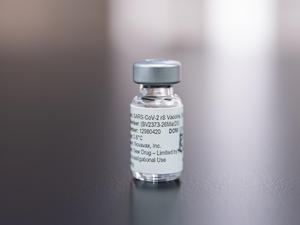
ReveraGen scored the Food and Drug Administration’s approval in late October for its treatment for Duchenne muscular dystrophy, or DMD, the most common and severe type of muscular dystrophy that plagues young boys with progressive muscle weakness and atrophy.
The therapy, called vamorolone, has anti-inflammatory properties similar to traditional corticosteroids such as prednisone — but without typical complications such as weakened bones and stunted growth.
For decades, the market has lacked a steroid “that’s able to keep the benefit, but mitigate some of the side effects,” said Eric Hoffman, president and CEO of ReveraGen.
The drug showed in clinical trials to improve muscle strength and stature in DMD patients, and otherwise was comparable in its effects to corticosteroids, the standard of care for DMD. It’ll be sold starting in 2024 under the brand name Agamree by Coral Gables, Florida-based Catalyst Pharma Inc., via a sublicensing deal with Switzerland’s Santhera Pharmaceuticals.
Under the agreement, worth potentially more than $350 million, ReveraGen is set to receive milestone payments, including $16 million upon the FDA’s approval last month.
Hoffman co-founded ReveraGen with Kanneboyina Nagaraju, its vice president of research, while leading the Center for Genetic Medicine Research at Children’s National under its then-chief academic officer, Dr. Mark Batshaw. There, they built up infrastructure for translational and clinical research in this area, Hoffman said. And they teamed up with chemist John McCall, ReveraGen’s vice president of chemistry, as the third co-founder to start the company in 2008.
To get to this point, they haven’t taken outside investment because many viewed their work as “a very unsexy program” when compared to the “shiny objects” like gene therapy, for instance, Hoffman said. “It was hard to get venture capital buy-in, so eventually we just said ‘OK, we give up — and let’s just see if we can make a go of it with nondilutive funding,’ which we were able to do.”
The co-founders secured a total $75 million in grant funding — from the National Institutes of Health, Department of Defense and European Commission — as well as donations from several nonprofits and foundations. They gave 40% of the company’s shares to Children’s National in exchange for the intellectual property they’d developed, and donated shares to D.C.’s Foundation to Eradicate Duchenne, which helped get the research going, Hoffman said.
“With this alternative business model, we never had cash in the bank,” Hoffman said. So the recent $16 million milestone payment marks “the first time that we’re stable in over 15 years,” he said.
Now ReveraGen is paying back its foundation partners and paying off its bills, Hoffman said, noting that in the short term, the priority is “making sure the ship’s sailing solidly.”
It’s unclear at this point how large the U.S. market opportunity could be for vamorolone, because it would need to replace the existing standard of care and is the seventh drug approved for DMD, Hoffman said. But the European Medicines Agency is expected to approve the drug in December, following a committee’s recommendation that it issue the nod, which would make vamorolone the first treatment for DMD approved in the European Union, “Uptake could be quite a bit more rapid there." Hoffman said.
Hoffman and his co-founders also see a larger opportunity for vamorolone. They’re running a clinical trial in Becker muscular dystrophy, a milder form of DMD. And because corticosteroids are the most prescribed drugs worldwide, vamorolone could play a role in the other disease areas where corticosteroids are often used — from multiple sclerosis and inflammatory bowel disease to epilepsy and brain tumors to arthritis and asthma, Hoffman said. Studying its use in such disease areas, he said, is “very much on the top of our mind.”
ReveraGen was the first spinout from Children’s National, and that work “paved the way for entrepreneurship at the hospital,” Kolaleh Eskandanian, vice president and chief innovation officer for Children’s National, said in a statement. That includes the creation of the pediatric nonprofit’s commercialization arm, Innovation Ventures, which fosters the development of medical products via such entrepreneurship.