
Bethesda health-tech company BrainScope Co. Inc. has launched a new tool to help doctors more easily assess and treat concussions — and is raising millions in new funding for the rollout.
The medical device firm announced Tuesday the launch of what it calls the Concussion Index, an algorithm cleared by the Food and Drug Administration that builds on its existing medical device’s capabilities. That product, a disposable headset and a handheld device that processes the wearer’s brain activity, helps evaluate suspected traumatic brain injuries within 72 hours.
The new feature tailored to concussions also expands that time frame to “any point in concussion assessment,” CEO Susan Hertzberg said in an email Wednesday.
BrainScope is now fundraising, shooting for about $15 million and expecting to close a transaction within 90 days, Hertzberg said. That capital will be used to speed up and expand the company’s commercial execution. She declined to disclose revenue or prior investments, but PitchBook pegged its total funding at more than $63 million as of April 2020. That includes previous venture financing from Revolution, DBL Partners, ZG Ventures, the Maryland Venture Fund and others.
BrainScope has also received about $30 million from eight Department of Defense research contracts.
BrainScope reports that its device cuts down on the need for CT scans in the emergency department by more than 30% — important both because it avoids radiation to the patient and gives a full picture of the injury, including severity, to the physician. The product uses artificial intelligence to produce immediate results, which enable doctors to better determine the likelihood of concussions or bleeding in the brain, for instance. It runs on batteries and requires no lab testing, taking less than 20 minutes to run and report results.
The neurotechnology company is now taking orders for the latest version of its device, which in addition to the concussion tool also includes a clinical assessment in pediatric head-injury patients and another concussion evaluation specific to the military. It requires a doctor’s order for purchase.
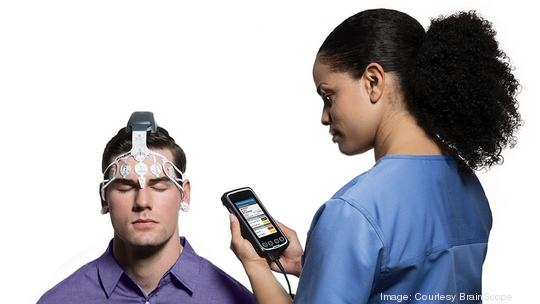
BrainScope received FDA clearance in September 2016 to sell its first product, which it developed with the Department of Defense to assess a range of traumatic brain injuries. It’s still the only medical device to receive the agency’s stamp “for the objective identification of the full spectrum of head injury from brain bleeds to concussions,” Hertzberg said.
The company’s most recent launch follows an FDA clearance in 2019 and multiple indication expansions for its device, which is used by the U.S. military, hospitals, urgent care and occupational health centers, concussion clinics, university sports programs and clinical trials. Its latest version aims to better assess the millions of U.S. patients who may suffer from concussions annually.
BrainScope has seen more opportunity during the pandemic, as hospitals look to become more efficient, Hertzberg said. “There are lots of head injured patients passing through the ED and in an infectious prone environment, we help move patients through the ED,” she said. “Nine out of 10 head CTs currently performed on head injured patients is negative for intracranial brain bleed. We help with efficiency and productivity by ruling out the likelihood of a brain bleed and reducing the need for unnecessary head CTs in the ED.”
The company is also exploring the prospect of using its algorithms for brain function to identify and track “long-hauler” Covid-19 patients with neurological symptoms, she said.
BrainScope, which moved from St. Louis to Bethesda in 2008, now counts 36 employees.
Hertzberg joined the company in June 2019 from Franklin, Tennessee-based Prelude Fertility, which merged with Houston’s Inception Fertility a few months prior. She was previously president and CEO of Boston Heart Diagnostics, which she led to its $200 million exit in January 2015, and CEO of French molecular diagnostics company Ipsogen, which then sold to Germany’s Qiagen. She’s also held positions with Quest Diagnostics, Abbott Diagnostics and Oxford Health Plans, now part of UnitedHealthcare.




