Casey Grage wants to change the way doctors drill holes into the skull — and armed with fresh funding, the neurotechnology startup founder is working toward launching her product to do just that and tapping research partners to get it out there.
The CEO of Hubly Surgical has raised $900,000 in an oversubscribed funding round, $300,000 more than she set out to secure in 2021, from backers including Canadian pre-seed investment vehicle First Fund, the round’s lead investor; Texas angel group SWAN Impact Network; and D.C.’s Halcyon Fund, which made a $50,000 investment.
This round brings Hubly’s lifetime funding to more than $1.3 million, following $300,000 from San Francisco’s Alchemist Accelerator and angel investors in early 2020, and another $140,000 in nondilutive capital from grants and pitch competitions.
“Boring into a person’s skull with a hand-crank drill is fraught with potential problems and adverse events," Grage said in an email. "Residents simply do the best they can with the tools and training they’re given today. Why not make the process as simple as it can be?”
The Hubly Electric Drilling (HED) system aims to enable doctors to more safely and effectively drill holes into the skull without damaging underlying tissue. That process is necessary in numerous instances, including for a condition called hydrocephalus, in which spinal fluid builds up or doesn’t absorb properly. It can happen in cases of traumatic brain injury, hemorrhage, aneurysm or stroke, among others — and can lead to brain damage or death if untreated. Addressing it requires drilling holes into the skull for a tube to drain the fluid. But the current procedure uses “antiquated” tools and can be “unnecessarily dangerous,” Grage said.
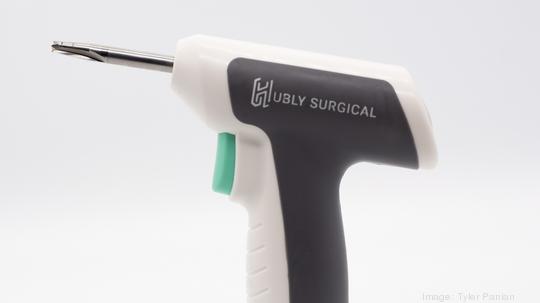
Hubly’s charge: improve quality of care and outcomes with the company’s electric drill. The new financing positions its five-person team to submit its application to the Food and Drug Administration for clearance. Hubly is working with Lisle, Illinois-based medical device design and development firm Ontogen Medtech to get it done.
The company is also looking for research partners to run cadaver labs, which would help establish standard operating and training procedures, and for clinical trials, which would aim “to show how the HED System can improve patient outcomes compared to today’s standard hand-crank drill as well as the extent physicians find the HED System easier to use,” Grage said. Hubly already counts Northwestern Medicine of Chicago and UMass Memorial Health of Worcester, Massachusetts, as research partners.
Also on the to-do list: hire a vice president of sales and marketing, and manufacture products ahead of landing that regulatory green light. Grage is shooting to submit that application by the end of the first quarter of 2022 and, per that timeline, would expect a response by the third quarter.
Hubly sees Level 1 trauma centers as well as community hospitals, rural clinics and ambulatory care sites as its future customers. But, Grage said, the company will first target urban teaching hospitals with large neurosurgery residency programs. The goal is to generate revenue from hospitals, and for those facilities to be “entirely reimbursed by insurance,” she said.
“We're starting off with our first goal of dramatically improving patient outcomes for this procedure while reducing dependency on operating rooms and cutting down on costs,” Grage said, adding that Hubly’s device could have other applications going forward.

Hubly, which also has an office in Chicago, previously went through the Halcyon incubator, the nonprofit’s flagship residency program in Georgetown, where Grage maintains office space. The investment in Hubly from Halcyon’s new fund, which Halcyon has not formally announced the existence of, comes soon after it also backed Arlington’s OxiWear, also an incubator alum — and a medical device company. We have reached out to Halcyon for comment and will update this post as we hear back.
Grage, who remains based in her native Fairfax County and holds a bachelor's in neuroscience from Northwestern University, started Hubly after taking a medical device entrepreneurship class at Northwestern's Kellogg School of Management. In that class, she met Dr. Amit Ayer, a neurosurgeon at the university’s medical school who was working toward his MBA. He became her co-founder and together, they invented the HED System.
“I’ve always been passionate about neurology because, like many, I have a long family history of Alzheimer’s and Parkinson’s diseases,” Grage said, adding that after working in a neurodegenerative research lab, she pivoted from her plan to pursue a career in neuroscientific research. “I decided I wanted to be closer to patient outcomes. I wanted a hand in both creating life-saving tech and aiding in the adoption of that tech into patient's lives,” she said.
Grage, age 22 — and one of our 25 Under 25 honorees of 2021 — previously worked as a software engineer at JPMorgan & Chase Co.
Click through the gallery below to see the rest of this year's 25 Under 25 class.
25 Under 25 of 2021
Age: 20
Title: CEO, Sa’akom International
By day, Calista Amrell is an executive assistant at D.C. engineering firm Sera Engineered LLC. By night, she’s developing Sa’akom, a nonprofit providing impoverished families in Cambodia with income and education through farming. The recent George Washington University graduate, who resides in Silver Spring, is leading her nonprofit to deliver its first farm, one she said will produce enough revenue to be self-sustaining once it reaches full capacity. On deck for the startup: starting construction in early 2022 on a full-scale agriculture facility. That’s while Amrell prepares to apply to graduate school for her doctorate in economics.
Amrell pulls from more experience than her two decades suggest. She grew up in a small town in Florida, surrounded by a community dependent on agriculture. She was raised by a father who runs a small business and two older brothers who now each have their own farms. She’s supported herself through college while advancing her venture — and completing her bachelor’s in political science in just two years. “Sa’akom has also allowed me to follow my own philanthropic interests,” she says. “The social programs we plan to implement with our full-scale farms have a tremendous capacity to make a positive impact on the communities in which we are located, and I’m very excited to expand our mission in Cambodia.”
Age: 19
Title: Founder, Ngoma Kenya
Sophia Andrews, part of American University’s class of 2023, is an international service major planning to focus on inequality and development in East Africa. She’s also in the school’s global scholars program, which positions students to graduate early and earn master’s degrees in their fourth years. Meaning Andrews isn’t wasting any time. The sophomore runs Ngoma Kenya, a nonprofit that focuses on arts education, orphan care and girls’ empowerment in Kenya. She started that business at age 15, after her first trip to Kenya. And as a dancer and performing artist, she says, “I believe the arts are critical and can be used to make a difference in our world. We strive to give the children in our programs a way to express themselves creatively and meet their basic needs.”
In four years, the company has grown from small ballet classes to serving kids across Kenya. Starting in summer 2020, that included the launch of a youth advisory committee that’s now building its next cohort. Beyond this work, Andrews interned on Capitol Hill with Rep. Lisa Blunt Rochester, a Democrat from Delaware, her hometown, as well as with the Delaware Center for Justice, where she focused on police reform within the state. This fall, she’s taking a gap semester and moving to Kenya to develop her team there.
Age: 22
Title: Founder, CEO and creative director, 3ird Eye LLC
Amir Barnett is a first-time entrepreneur with a big vision: to bring a fresh perspective to the art world. With his art and streetwear brand, 3ird Eye LLC, Barnett is juggling multiple projects slated to go live in the coming months, including a couple in the nonfungible token (NFT) market. The business blossomed “as a direct response to my mental health,” he says after having lost both his father and a friend during the Covid-19 pandemic. “To clear my mind and express feelings and emotions I didn’t want to verbalize, I began expressing myself through art. Combining beautiful colors, symbolism, and words of positivity and optimism on canvas pulled me out of a dark place mentally and served as the foundation of what has become 3ird Eye.”
The Chevy Chase founder — who works as a business analyst for Deloitte Consulting and holds a bachelor’s in biological engineering from North Carolina Agricultural and Technical State University, a flagship historically Black college and university — has taken that talent to larger platforms. He recently finished painting his first mural, a 160-square-foot piece in Miami. He’s also preparing for his own art show in the D.C. area, showcasing a mix of paintings and digital NFTs. But the nascent business remains a major focus. Barnett is eyeing $10,000 in sales by the end of its first year, while expanding the brand’s presence into major fashion and art markets.
Age: 23
Title: Co-founder, Moonvu
For Thaddeus Bayston, a post-high school gap year to pursue his startup turned into a mission — to build a company that helps level the playing field for investors by democratizing access to data. His Alexandria startup, Moonvu, provides a platform to connect transparency advocates, known as data farmers, with investors. The company analyzes more than 3 million pieces of data each day, counting products created at a factory, for instance, or foot traffic at a retail store. It then uses artificial intelligence and machine learning to break that information down into practical, actionable insights for individual investors. He intends to make such information both as affordable and accessible for independent investors as it is for Wall Street.
That started this summer, when Moonvu released production data by model from Tesla’s plant in Fremont, California, before the company's own report was released. While Moonvu’s genesis lies in the Bay Area, Bayston said he had a tough time scaling and competing for talent there, so he moved the company to Greater Washington where, he said, he found “a large network of like-minded people who actually want to engage, with ample access to capital and talent.”
Age: 24
Title: Founder, Button Helper
Robert Bolen’s startup, Button Helper, works to help people with disabilities easily dress themselves, thanks to the use of magnetic buttons. The Georgetown University McDonough School of Business grad started the company nine years ago for a friend who struggles with cerebral palsy. He’s since garnered recurring wholesale orders from giants like Sunovion Pharmaceuticals Inc. and Amazon.com Inc.
Button Helper took home a $30,000 prize at Georgetown University’s Bark Tank pitch competition in October 2020, after launching on Zappos and working with MedStar Georgetown University Hospital neurologists to develop the product for people with arthritis, Parkinson’s disease, movement disorders and joint pain. The business just wrapped up a pilot program at the Polytrauma System of Care at Hunter Holmes McGuire VA Medical Center and filed for its fifth patent for a more durable, thinner iteration of its product. That’s on tap to go live this fall.
Age: 21
Title: Co-executive director, Technica
Hugo Burbelo is studying computer science and philosophy as part of the University of Maryland, College Park’s class of 2022. That’s while helping to run what he and fellow honoree Utsa Santhosh bill as the largest hackathon in the world for underrepresented genders in tech. That program, called Technica, brings thousands of students to campus each year for a weekend of inclusive learning, growth and exposure to the industry. It involves running a 124-student team and spearheading the annual gathering.
The pandemic led the duo to take the event virtual in 2020 — and to build their own custom platform for it, from the bottom up, to support the event “and ensure that participants would still experience the full magic of Technica despite the virtual format,” they say. And it worked. They turned that platform into a customizable software-as-a-service product that, now, other organizations can use for their own events. It won second place and $10,000 — and the audience choice award — in the University of Maryland’s 2021 Pitch Dingman Competition.
Age: 25
Title: Owner, Blackbird Salon
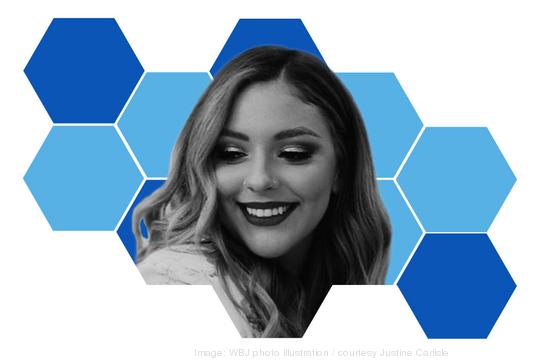
The pandemic led plenty of entrepreneurs to shut down. Justine Carlisle decided to open up. With partner and fellow honoree Devin Cook, she established the eight-chair Blackbird Salon in the District’s Navy Yard neighborhood during 2020. The hair color and extension specialist — and now, business owner — is now working to grow the business and her team of stylists.
Carlisle started her hair career at age 18, graduating from Aveda Institute Washington, D.C.’s hair school in 2014. “Opening a brick-and-mortar business during a pandemic at 24 with no college degree has been the hardest, but most rewarding, experience so far,” she says. “I really look forward to growing it, investing in my employees and developing great leadership skills with my partner.”
Age: 25
Title: Co-owner, Blackbird Salon
Devin Cook teamed up with fellow honoree Justine Carlisle to open the Blackbird Salon in D.C. — in the heat of the coronavirus crisis. Today, the duo has a team of more than 15 employees and a space for working women in the city. But the work is more than a shop.
When she started her entrepreneurial journey, she struggled to find young mentors to guide her. So now, she says, “I am on a path to inspire young women and professionals that you can find success and stability without a college degree.” That, she says, means finding mentors and leaders “who shed light on ways to find careers in industries that were once known as ‘not enough’ or ‘long term.’”
Age: 22
Title: CEO, Hydrova
Julian Davis started his company in the Georgetown Venture Lab in March 2020 with co-founder Rostam Reifschneider. They’re both now working full-time on Hydrova, which is developing a low-cost and zero-emissions tech for making hydrogen. Since its inception, the clean energy startup has received grant funding from the Sandbox Innovation Fund at Reifschneider’s alma mater, the Massachusetts Institute of Technology; it’s earned a $20,000 prize at the University of St. Thomas’ e-Fest pitch competition; and it is now going through CleanTech Open’s startup accelerator program.
In the coming months, the co-founders plan to raise their first round of capital to expand their team and start working on a commercial prototype of the tech. And Davis, who holds a bachelor’s in management and physics, is considering returning to D.C. following that round, after relocating to his family’s home in San Diego through Covid-19.
Age: 22
Title: Founder and CEO, Hubly Surgical
Casey Grage dove into neuroscience in 2015 and never looked back. She researched Parkinson’s disease at George Mason University’s Computational Neuroplasticity Lab, conducted mice surgeries and electrophysiology experiments via two-photon lasers at Northwestern University’s Kozorovitskiy Lab and the list goes on. Grage earned her bachelor’s in neuroscience from Northwestern and previously worked as a software engineer at JPMorgan & Chase Co.
Her ambition bloomed at a young age. She self-published a children’s science fiction novel at age 10, successfully campaigned to install solar panels in Fairfax County Public Schools for the next decade and, most recently, started and now leads Hubly Surgical. “I knew the unmet need of antiquated intracranial access resulted in thousands of people needlessly dying every year in the U.S. alone. I wanted to do something about it,” Grage says. She’s the inventor behind the neurotechnology startup with a system for safely drilling holes through the skull without damaging underlying tissue. Her company, part of a fellowship at Georgetown incubator Halcyon, is now preparing its application for clearance from the Food and Drug Administration, on track to submit that by year’s end.
Age: 25
Title: CEO, Map-Collective Inc.; president, Anamakos LLC
Tara Gupta is juggling school with not one, but two businesses. After earning her undergraduate degree from the Rhode Island School of Design in 2019, she’s now in the Georgetown University McDonough School of Business class of 2022. That’s while leading Map-Collective, a clean-tech startup with a carbon-tracking and supply chain platform that lists carbon footprints by companies, governments and individuals. Clients range from the government of Maui to cannabis company Curaleaf.
At the same time, Gupta is president of real estate development startup Anamakos, which is focusing on building sustainable communities and infrastructure. That includes a 40-unit development in D.C., the first in a series of projects it’s planning in the region in partnership with Fillat Architecture. Anamakos is using the District as a model city before expanding, Gupta said. She was recently named to Forbes’ Next 1000 Entrepreneurs list for 2021.
Age: 21
Title: Founder and CEO, OTGbaby Inc.
Brett Guterman started Rockville-based OTGbaby to change the way parents change diapers. The company’s product, called The Go Bag, is a patented diaper-changing backpack that allows parents to change their baby anytime, anywhere. The 3-year-old business grew from a concept from a high school entrepreneurship program at the Bullis School in Potomac that Guterman brought to college at George Washington University’s School of Business, where he’s set to graduate in May with a major in finance.
The local entrepreneur leads the startup with his mother, Barbara, who’s now doubling as his business partner. And Guterman is leading the charge, now looking to grow the company’s online retail presence on its own website and on Amazon.com, while broadening its existing wholesale relationships in 30 boutiques across the country — and bringing on more. That’s before introducing new patented designs and add-on products by year’s end. OTGbaby just completed GWU’s Summer Startup Accelerator program and is targeting big growth in Greater Washington, its founder’s hometown.
Age: 25
Title: Chief technology officer, Column PBC
Leo Hentschker leads the software engineering team at Column, a public benefit corporation with a tech platform that modernizes the placement of public notices — from public meetings and foreclosures to legal proceedings and government updates. Since launching in September 2020, the company has gained traction in more than 11 states and has partnered with some of the country’s largest media businesses.
The D.C. entrepreneur was an early software engineer at Quorum Analytics, the predecessor to Column. Like Column’s founder and CEO, Jake Seaton, a 25 Under 25 honoree of 2020, Hentschker dropped out of school at Harvard University to help build the company. He then returned to college and graduated in 2019 — in three years — with a degree in math.
Age: 24
Title: Program manager, Georgetown Entrepreneurship; founder, Greenfin
David Lange has two passions: helping young D.C.-area entrepreneurs and creating a more sustainable food system. Now part of Georgetown University’s MBA flex program, through which he’s pursuing a certificate in sustainable business, he’s simultaneously working as program manager for the Georgetown Entrepreneurship Initiative in the university’s McDonough School of Business. As part of that work, he’s building out its Georgetown Startup Interns program, which allows its undergrads to work part-time at a startup and with a licensed career coach while, too, earning course credit through skills workshops.
The work doesn’t stop there. Lange is also publicizing his new book, “A Farm on Every Corner,” about changes needed in the American food system. Lange’s startup, Greenfin, aims to be the first in the world to commercially farm Chilean sea bass. To do it, it’s using a land-based recirculating system, to raise the fish with a smaller environmental footprint than traditional aquaculture operations. The company is preparing to start courting angel investors, he says, with eyes toward starting construction on its pilot-scale facility in 2022.
Age: 20
Title: Founder and CEO, Zfluence
Ava McDonald is the mind behind Zfluence, a marketing startup that arms companies with the tools and insights to attract new Gen-Z customers. Oh, and she started the business at age 17. That, McDonald says, “was the single most empowering thing that I’ve ever done.”
That made the D.C. entrepreneur a champion of teen entrepreneurship while she works toward her English major as part of Georgetown University’s class of 2024. “My advice to members of Gen Z who are thinking about starting a business is to do your research to confirm that there’s a market opportunity for your business, and make sure that you’re willing to put in the hard work,” she says. “Then go for it and don’t look back.”
Age: 22
Title: Co-founder and CEO, Blimp Logistics Inc.
Camilo Melnyk co-founded and runs College Park drone delivery startup Blimp Logistics, which is building a decentralized drone delivery network to provide last-mile delivery services to rural and suburban communities. The venture, which he runs with fellow honoree Spencer Yaculak, uses autonomous drones and automated hubs to deliver packages up to 30 pounds from local grocery and convenience stores to customers’ homes. The goal: bring down the cost of delivery services and address the lack of affordable access to grocery stores from which many suburban and rural residents suffer.
The 2-year-old company took home the $30,000 grand prize in the quattrone venture track at the University of Maryland’s Pitch Dingman Competition. And that money allowed the entrepreneurs to bring on new team members while developing the hardware and software needed to power and grow its drone network. Melnyk graduated from the University of Maryland in May with a bachelor’s in aerospace engineering. He’s also a volunteer firefighter in College Park.
Age: 21
Title: Founder and CEO, Social Currant
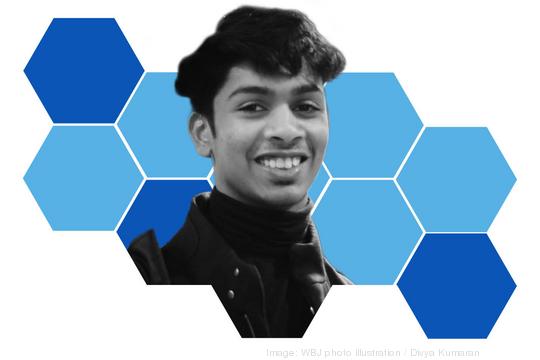
Ashwath Narayanan moved from the U.S. to India when he was 3, and didn’t return to the U.S. until college in 2019. He’s now a senior at George Washington University studying journalism. But in India, he worked with multiple organizations before deciding to launch one of his own. Enter, Social Currant.
The D.C. creative agency uses emerging media to help social impact organizations reach young people more effectively. That work involves helping those players leverage content through platforms including TikTok, Instagram, YouTube and Snapchat. In less than a year, it’s grown to 15 employees, more than 50 clients, a network of 200 influencers and tangible impact. Outside of Social Currant, Narayanan is a senior adviser to DC ArchAngels, a local angel investing group, in addition to advising and mentoring multiple startups across the country.
Age: 23
Title: Founder and CEO, Remora Inc.
Omar Negrón Ocasio works as a property accountant at NASA’s Goddard Space Flight Center in Prince George’s County, armed with a bachelor’s degree in accounting and human resources from the University of Puerto Rico. He’s now pursuing a master’s in public administration at George Washington University. But he has more on his plate, namely leading his startup, Remora, which provides clean and potable water through advanced technologies and products to communities in need, while promoting sustainable living and protecting the world’s water sources. Its chief product: a long-term water filtration device, powered by solar energy, that provides a sustainable source of clean water. The company puts those devices in communities that lack access to clean water, now with five already running in Puerto Rico and more planned for Latin America as the venture expands.
The D.C. entrepreneur grew up in Corozal, a small town in the mountains of Puerto Rico. When Hurricane Maria destroyed that world Sept. 20, 2017, everything changed, he says. “I lost everything that day. No home, no cars, no electricity and no water.” He and his family lived in a shelter for weeks, where the water supply wasn’t enough, forcing them to wait daily for a water truck. “After waking up all the time to the same issue, and after spending five months without clean water, I decided to use my struggle as inspiration to develop a long-term solution for the water issues Puerto Rico was facing at the time.” Remora’s first prototype has given more than 6 million gallons of water to more than 500 people. And it’s garnered awards from organizations including T-Mobile, Ashoka Foundation, Clinton Foundation and Ford — with Ocasio taking first prize in the social venture track for GWU’s 2021 New Venture Competition.
Age: 25
Title: Co-founder, Sign-Speak Inc.
Yamillet “Yami” Payano is co-founder and chief operating officer of Sign-Speak, a software service that uses machine learning to translate American Sign Language to English, and vice versa. She’s now developing its fundraising and marketing strategy for the startup, after serving as a quantitative analyst for Fannie Mae’s fair lending team. And she has some help, now with financial backing and other support from Google for Startups’ Black Founders Fund.
As a first-generation, low-income student with parents who didn’t speak English or obtain a formal education, “I have been my parents’ translator, my younger sister’s educational adviser and the family accountant for as long as I can remember,” Payano says. She had to learn on her feet. “In the Dominican Republic, I grew up to the sounds of ‘el gallo pelón’ — the ‘bold rooster’ — chickens running around the yard, and my mother’s constant reminders to be in the house before 6 p.m. to avoid being out when rival groups were engaged in sunset shootouts. I never really had a childhood, and I believed that with my background, I would not be able to attend college,” she says. But she secured a Gates Millennium Scholarship to pay for school, and graduated from American University with a bachelor’s in economics and math, and a minor in Mandarin — all while building her own startup.
Age: 24
Title: Programs manager, Black Girl Ventures
Maria Pope joined Black Girl Ventures in October 2020, with a bachelor’s in business and economics from Texas A&M University. She quickly moved from her post as partnerships and outreach manager to community success manager and, this past April, was promoted to program manager. In that current role, she oversees the organization’s national fellowship for Black and brown women. That includes founders in a handful of cities across the U.S. and arming them with the skills and tools needed to create more equitable entrepreneurial ecosystems in their markets. Among that work has been helping to manage BGV’s partnership with Georgetown social entrepreneurship nonprofit Halcyon, among other initiatives.
BGV, led by Shelly Bell, has fast become a national name, evolving from a modest District nonprofit to a multicity effort to fund, support and promote female founders of color. It’s partnered with big brands such as Nike and Warby Parker, and hosts pitch competitions in cities across the country, from D.C. to Houston to Philadelphia to Miami.
Age: 20
Title: Co-executive director, Technica
Utsa Santhosh leads Technica, the largest hackathon in the world for underrepresented genders in tech, as a student at the University of Maryland, College Park. There, she’s pursuing a degree in computer science, set to graduate in 2022. She runs the 124-person student organization with fellow honoree and classmate Hugo Burbelo, with whom she led the initiative through a massive pivot during the pandemic.
Technica, which brings thousands of students to campus each year for a weekend of tech training, couldn’t happen in-person in 2020 because of the coronavirus crisis. So Santhosh and Burbelo made the switch to virtual, building their own custom platform from scratch — a gamble that, they say, paid off. The partners turned that platform into a customizable software-as-a-service product that, now, other organizations can use for their own events. It won second place and $10,000 — and the audience choice award — in the University of Maryland’s 2021 Pitch Dingman Competition.
Age: 21
Title: Founder and CEO, DopaMe
Tian Shi started DopaMe after learning her grandfather suffered from Alzheimer’s disease — and discovering that preemptively catching neurodegenerative diseases can drastically increase the overall quality of life for the one in six people affected by these diseases worldwide. So she set out to develop a dopamine sensor that can detect and monitor internal dopamine levels. “I hope this novel technology can detect early imbalances in dopamine levels to mitigate the impact of these neurological diseases that are breaking apart millions of families worldwide,” she says. “Hopefully, one day, my grandfather can use my technology and remember who I am.”
Outside of DopaMe, Shi is majoring in biology, minoring in cognitive science and working toward a certificate in entrepreneurship as part of Georgetown University’s class of 2022. She’s spent the summer as a student researcher working with a Georgetown professor to create a tool for international governments to gauge their national risk for biohazards and infectious diseases, while working at two local startups: Danya Sherman’s KnoNap and Shavini Fernando’s OxiWear. That came after she wrote and published a book, “Exceptionally Average,” in which she chronicles the stories of women, immigrants, people of color, and high school and college dropouts who pursued entrepreneurship.
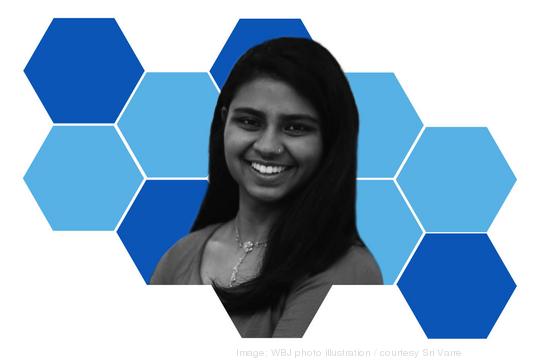
Age: 21
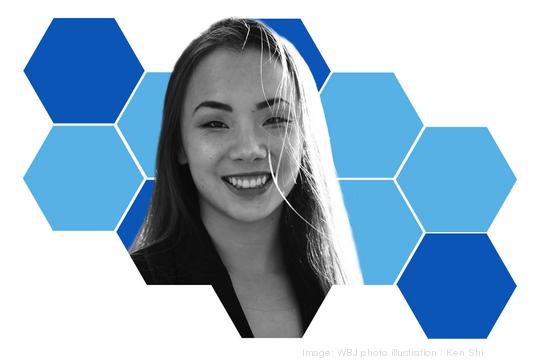
Title: Product development coordinator, The Lip Bar Inc.; fellow, Venture for America
Sri Varre has had a busy year. She graduated as part of George Washington University’s class of 2021 with a bachelor’s of science in international affairs and economics. She moved to Detroit for a two-year entrepreneurship fellowship program — to get startup and leadership experience — with New York’s Venture for America. And she’s working on the product development team for vegan beauty brand The Lip Bar, which focuses on diverse and inclusive representation in that space.
But her base is D.C., where she plans to return and continue working part-time as a business development associate for District creative agency Social Currant. That’s all after participating in GWU’s first Summer Startup Accelerator in 2019, where she worked with three teams, including 2020 honoree Viva Vita LLC, and interning with the Vinetta Project and former 25 Under 25 winner KnoNap. Long-term? She wants to use social entrepreneurship to promote gender equality.
Age: 23
Title: Founder and executive director, Sikhona Rescue Centre
Elli Wachtman started and leads Sikhona Rescue Centre, a grassroots social enterprise that aims to provide a physically and psychologically safe space for survivors of emotional, sexual or physical violence. Now completing its registration as an international nonprofit, Sikhona looks to empower families to transform gender norms in Kenya. “Undecided for the first two years of college, I wrestled with how to pair my knowingly analytical mind with my heart for people,” Wachtman says. “I had known since I was young that I would spend my life leveraging my privilege to lift the marginalized. Collaborating with and empowering others is where I have always found my truest purpose. I simply didn’t know which path would lead me to foster the most meaningful, effective and lasting change.”
But when she helped create a community engagement scholarship program while pursuing her bachelor’s in mathematics at Capital University in Columbus, Ohio, she found her calling in leadership through service, she says. And in 2018, her move to Kitengela, Kenya, sparked the idea for Sikhona. Now, she’s working toward a master’s in international development and certificate of social innovation from American University.
Title: Co-founder and head of mechanical design, Blimp Logistics Inc.
Age: 23
Spencer Yaculak is co-founder of Blimp Logistics, a College Park drone delivery company that’s bringing last-mile delivery services to rural and suburban communities. How, exactly? It involves building a decentralized drone delivery network using autonomous drones and automated hubs, which it’s now building out. The model involves delivering packages up to 30 pounds from local grocery and convenience stores directly to customers.
The Bowie entrepreneur runs the startup with fellow honoree and former classmate Camilo Melnyk. Yaculak is studying mechanical engineering at the University of Maryland, where she and Melnyk led their 2-year-old business to a $30,000 grand prize at the school’s Pitch Dingman Competition. “I love engineering, and since 2016, I’ve held five different internships in my field," she says. "But I also love to ride: I’m a seasoned longboarder, and I’ve been shredding down mountains for almost a decade now.”