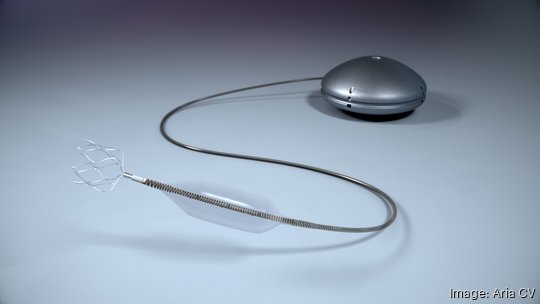
A St. Paul-based med-tech company celebrated Valentine's Day with the first implant of its second-generation heart implant device to treat pulmonary hypertension.
The Gen 2 Aria CV Pulmonary Hypertension System — which mimics the function of a healthy pulmonary artery, without using any batteries, pumps or electronics — was implanted by doctors Ashwin Ravichandran and Scott Hittinger at St. Vincent Cardiovascular Institute in Indianapolis.
Aria CV CEO Dan Gladney said the patient is recovering well and seeing improvements already.
The Valentine's Day implant was conducted as part of an early feasibility study. The FDA has approved 15 patients for the study; if that goes well, the company hopes to expand the total to 30 patients, Gladney said.
"This first patient with the implant was on supplemental oxygen when needed," Gladney said. "After we implanted this patient, she told her doctor she is breathing better. That, to us, is huge."
Ten hospitals, including the University of Minnesota, are part of the early feasibility study.
In 2022, the company raised a $13 million bridge round to develop the generation 2 product, which is much stronger and smaller than the original design, Gladney said.
"We believe that our tech will have a direct impact on right ventricular function," Gladney said. "The implant allows the right heart to function better and recover over time and the patient will have a much higher quality of life."
The company plans to finish its early feasibility study by mid-2025 which will help it know what it needs to do during the clinical trial, which is projected to start in late 2025, Gladney said.
"What we are really looking forward to is completing series c round funding in the near future and opening a larger clinical trial in the U.S.," Gladney said.



