
Francis Medical, Inc. has reached a new milestone for treating prostate cancer. The secret? Water.
The Maple Grove-based startup’s Vapor 2 study has successfully treated one U.S. patient who had prostate cancer using water vapor technology, a process that avoids debilitating side effects that come with other prostate treatments like radiation. The study will treat 235 patients across 30 U.S. clinical sites – including Mayo Clinic, University of Minnesota and Minnesota Urology – and Francis Medical will follow the patients for five years and publish data about their progress.
The technology was discovered by Michael Hoey, founder and chief technology officer of Francis Medical. After his father died of prostate cancer, Hoey resigned from his academic life to develop new treatment technologies.
He was working on a car one day when water was condensing on his hand. Hoey thought “I might be able to ablate tissue with this,” according to Mike Kujak, president and CEO of the company. That set the course for his new career.
In mid-2025, Francis Medical will submit a 510(k) to obtain FDA approval and begin marketing the technology in the U.S. In 2027, the startup will seek more advanced regulatory approvals and could be the first prostate cancer product of its kind on the market with a PMA – or premarket approval – label.
Dr. Arvin George from Johns Hopkins and Dr. Samir Taneja from NYU Langone are co-principal investigators in the study, and the first patient procedure was successfully completed by Dr. Naveen Kella of The Urology Place in San Antonio, Texas.
In total, more than 40 men have been treated with the technology so far, between early feasibility studies, the current clinical study and international efforts.
The technology works by heating sterile water, injecting it into a needle, putting the needle into the urethra to reach the prostate and creating a small lesion that ablates – or removes – the cancerous cells. It can be completed in under an hour or even under 20 minutes.
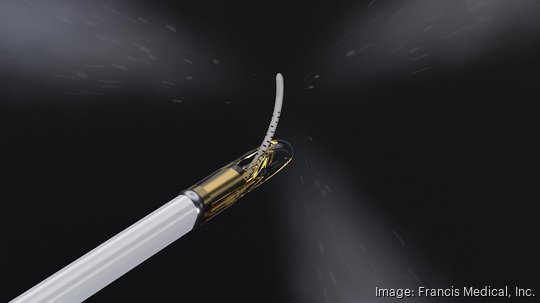
It doesn't include long hospital stays and avoids the debilitating side effects like incontinence, impotence or difficulties controlling bowel movements, which are common with other treatments.
“It’s nothing more than water,” Kujak said.
The startup was founded in 2018 as a spinout of NxThera Inc. when that company was bought by Boston Scientific in a deal worth about $400 million. It raised $25 million in series A funding, which was used to build the first-generation tech and conduct an early feasibility study. Once Francis Medical proved the technology was safe, it raised $55 million in series B funding to develop its second-generation product being used in the Vapor 2 trial.







