Medical device company NeuroOne Medical Technologies Corp. is coming out of a transformative year.
The Eden Prairie-based company raised $12.5 million, which allowed it to uplist from being an over-the-counter stock to trading on the Nasdaq by late May.
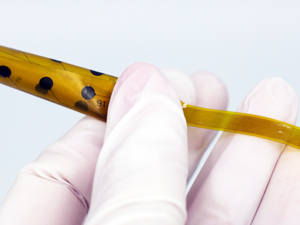
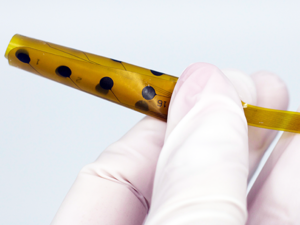
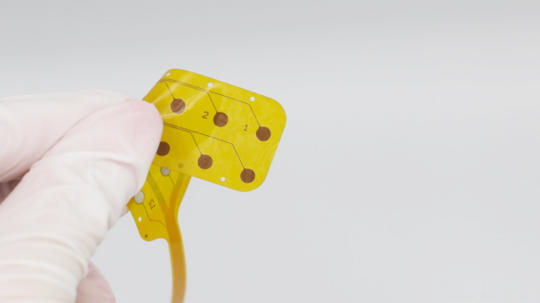
Then in September, it received FDA 510(k) clearance for its Evo sEEG Electrode that temporarily records, monitors and stimulates the subsurface of the brain with electrical signals. The technology is used for brain-mapping procedures in patients suffering from Parkinson's disease, epilepsy and other neurological disorders.
Now that the quiet period is over, NeuroOne CEO Dave Rosa talked to Minne Inno about the company's high points and what it's developing next. The conversation has been edited for length and clarity.
What stands out for you as a highlight of the past year?
One of the biggest things was the launch of our first product. Commercialization really started in the January timeframe. So getting to the point where you're able to commercialize your first product is really a huge milestone for an early-stage company.
Why make the move from trading over-the-counter to Nasdaq?
We were on the over-the-counter exchange since 2017. Being able to hit all the requirements to finally get yourself on Nasdaq, which is really where the big boys get to play, was critical. And when you get to Nasdaq, it opens up the door for a more sophisticated investor, whereas many institutional investors shy away from companies that are on OTC or other exchanges. So the ability really to raise capital with much more sophisticated investors, and a wider pool of investors was really critical.
Can you explain the difference between the two products that have received FDA approval?
When we when we originally started the company, we felt we could develop a higher-performing electrode technology that was more brain friendly. What we learned fairly early on is that it also had capabilities of not just performing diagnostic functions, but also therapeutic functions. What we have today is electrode technology for diagnostics. But we also have therapeutic combination devices, meaning you can perform both a diagnostic evaluation as well as the actual therapeutic procedure using the same device. Moving toward combination devices is better for the patient, because they only have to undergo one surgery.
How has hiring been? Have you been able to find enough talent?
I just literally had a compensation analysis done. And they said look, with the hiring environment being so difficult, there's more jobs than there are people willing to work. Instead of paying in the 1/3 bracket, you need to really target the center bracket at a minimum. This is the first time I've ever heard an assessment saying across the board you're going to be spending more for human capital than you have in the past. The good news is people that we hired so far are people that we've known from past companies, or that we had experience with before, so it hasn't been as difficult. But as we ramp up the company, next year and beyond, we're gonna run out of people. And I think we're going to be more subject to some of the challenges if the environment doesn't change.
What excites you the most about the year ahead?
It's really starting to ramp up sales with existing products. Second is, we not only have the chance to offer a diagnostic product, but we will expect to be submitting at sometime late next year for our combination diagnostics and therapeutic product. That's where it gets exciting for me and that's the next chapter in our history.