A Duke University spin-out has landed a federal grant to support the development of an at-home diagnostic test for multiple infectious diseases.
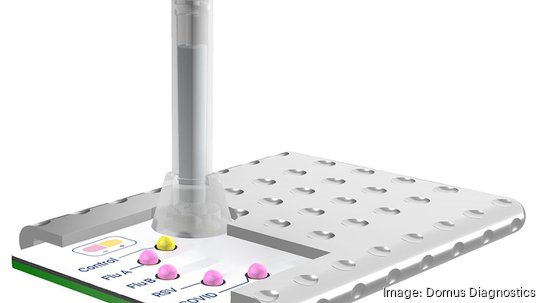
Domus Diagnostics has secured a $2.4 million contract from the National Institutes of Health that will help the startup prepare its testing platform for clinical trials. Domus is developing a low-cost detection test for the simultaneous identification of Covid-19, RSV and influenza A and B.
The award is a big step for the startup, which has been supported since its formation in 2021 by two seed rounds totaling about $2.1 million, said CEO Paul Chapman. During that time, Domus has focused on creating a device that's easy to manufacture and optimizing the chemistry behind it.

Much of this work has taken place at lab space, which the startup is doubling the size of, on Davis Drive in Research Triangle Park. Domus consists of five employees and contractors including two employees based in RTP, with another starting soon. This team includes Xin Song, the startup's co-founder and chief scientific officer, who is also a researcher at Duke.
The startup's other two co-founders include Duke professor John Reif and experienced biotech executive Harald Casebourne Stock, who serves as the executive chair of Domus' board of directors. A conversation between Reif and Stock while riding a ski-lift in Utah led to the formation of the company based around the idea of developing a low-cost, at-home test that doesn't require a power source. Stock later reached out to Chapman about joining the startup – the two previously worked together at the global pharma company Roche.
In its initial stages, Domus was focused on developing a simple Covid-19 test that could be made available in countries where testing accessibility was limited, said Chapman. But as the pandemic began to wane, the company pivoted and created a multiplex diagnostic with additional applications. This led to the company's existing efforts to develop a low-cost, at-home test for certain flu types, Covid and RSV, which Domus projects could be manufactured for $4 each.
"The foundation of the company was built on accessible testing," Chapman said.
The focus on a device that could make infectious disease testing more affordable and accessible globally helped Domus secure its grant from the NIH through the agency's Rapid Acceleration of Diagnostics program. In addition to the funding, this program opens up other sorts of NIH resources to Domus, like clinical samples that would otherwise cost money.
The program is intended to support Domus to the point where it's ready to enter its device into clinical trials. Chapman said the NIH program outlines a one-year period, but the company intends to move up that timeline and begin clinical trials in as early as six months.
The startup will need to secure additional financing to run the studies. Chapman said the top priority is non-dilutive sources, such as government grants or money from NGOs. Domus could also look to secure a strategic partnership with another company that may want to play a role in the clinical development. Lastly, the company could look to raise a Series A round.