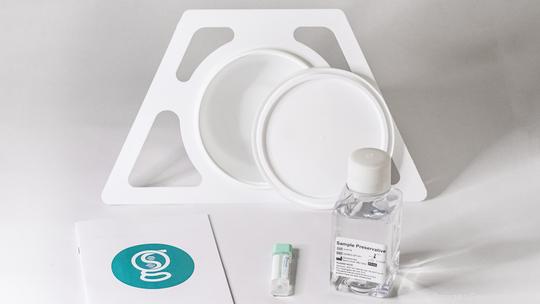
St. Louis gastrointestinal health startup Geneoscopy is taking steps toward commercializing its at-home, preventive screening test for colorectal cancer after a clinical trial yielded strong results.
“We’re rearing up to start commercializing,” said Erica Barnell, co-founder and chief science officer of Geneoscopy.
Genesocopy last week released results of its clinical trial, saying the study found its stool-based test “can accurately detect if people have cancer and if they have advanced adenomas that put them at higher risk of developing cancer.” Results reported by Geneoscopy said the study found its test had 94% sensitivity for detecting colorectal cancer and 45% sensitivity for detecting advanced adenomas.
Geneoscopy in 2021 started enrolling patients in its CRC-PREVENT clinical trial and that same year closed on $105 million in financing to advance its technology. Barnell said Geneoscopy’s clinical trial eschewed the traditional model of establishing sites that utilizes doctors to help recruit participants and instead used a “decentralized recruitment strategy” that allowed it to enroll a more diverse patient roster. Its trial included 8,289 individuals, with participants from all of the lower 48 U.S. states.
“The fact that we achieved such a high level of accuracy in a real world patient cohort kind of further demonstrates what we can do as a dialogistic (tool) and improved over current tests that are out there,” said Barnell.

Barnell said that 66% of the individuals in Geneoscopy’s clinical trial did not have a colonoscopy scheduled when they signed up for the study, which she said highlights the importance of at-home screening tools for colorectal cancer.
With its clinical trial results, Geneoscopy this month plans to submit its premarket approval application to the U.S. Food and Drug Administration. Premarket approval is a key step for commercialization and involves the FDA reviewing the safety and efficacy of medical devices. Premarket approval is required for companies to be able to market their devices.
The submission to the FDA isn’t the only step Geneoscopy is taking as it eyes commercialization. It is also finalizing a peer-reviewed article detailing its clinical trial results to publish this year in a scientific journal and engaging with the Centers for Medicare & Medicaid Services to seek out approval of its test for reimbursement.
Geneoscopy, founded in 2015, has 50 full-time employees. It has employees in 12 states, with about 60% of its staff located in the St. Louis region.