Two years ago, in the early days of a novel coronavirus spreading to almost every corner of the planet, it felt like something of a race. The planet’s best scientists were trying to keep up.
“When the pandemic started, one of the things that became imminently clear was that we were way too slow at diagnosing it and determining if somebody had COVID-19,” said Neal Woodbury, vice president of research and chief science and technology officer with Arizona State University’s Knowledge Enterprise.
In the pandemic’s early months, health care in the U.S. and other nations had stumbled over the scale, speed and accuracy of testing for the virus. “We all realized there wasn't going to be one set of solutions. You weren't going to solve this one way,” said Woodbury. Testing for the virus — especially prior to vaccinations being available — was a key component in containing it.
The “magic wand wish” was for simple, fast, cheap and reliable testing tools that could be put to use in crowded places like airports, theaters, concerts — anywhere people might gather quickly in large numbers. So-called “point-of-need” testing.
Scientists within Arizona State University pulled together a huddle to share ideas.
“What we wanted to accomplish was to create tests that allow people to detect early infection so they can gather with confidence that they're not going be an environment where there's potential transmission,” said Jennifer Blain Christen, who leads ASU’s BioElectrical Systems and Technology group.
It was a tall order. Biotech firms, government health agencies and universities around the world have been running together in this marathon for more than two years. ASU’s group won strong financial support from Arizona’s Department of Health Services in September 2020. The money has been put to work in three silos of virus testing innovation.
Streamlined testing
Blain Christen is an electrical engineer. She and her team now have working prototypes of small, portable test machines. They begin with a small amount of saliva, which is pressed through a flat plastic cassette. Wells in the cassette pull the saliva into chemical reactions. Within minutes, a scanner reads the outcomes as positive, negative, or in error. Picture the device inside a doctor’s office, or at the entrance of a large meeting.

“We could simply ask [attendees] to come about 40 minutes early, provide a saliva sample and have high-quality testing that detects early infection,” said Blain Christen. “Before you have any symptoms, before an antigen test would be effective, we can detect those early indicators of COVID-19. That allows people to gather, and they’ll be in a safe environment.”
Work continues to make the devices even smaller, more streamlined, and modular.
Microfluidics testing
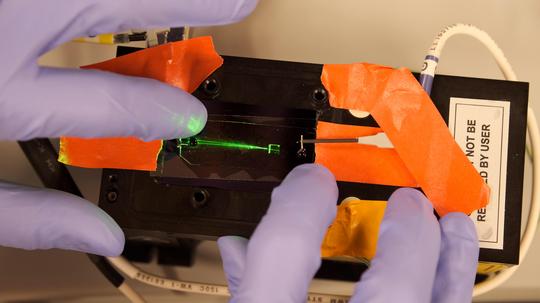
Mark Hayes is a molecular scientist. He and his team have poured their efforts into solving the testing problem through what’s called microfluidics. Think of it as sorting viruses and particles through flat, clear plastic slides, then hitting them with lasers and electrical currents. Hayes explains the big idea with a familiar image: the gigantic beam of light that shines from the Luxor Hotel pyramid in Las Vegas.

“You can see individual insects flying around in that beam of light,” Hayes says. “It’s a similar idea here. We have a little microfluidic device; we’re shooting a laser on it. If there’s a particle there — we filter out everything else but the coronavirus particle — it’ll scatter a little bit of light, and we can pick that up on our cameras.” Chips for sorting the virus particles can be made inexpensively, and on a massive scale.
High-accuracy testing at scale
Alex Green is a biomedical engineer, now at Boston University’s College of Engineering. Green and his team drove new work in lab-ready diagnostic tests. Their role was converting an assay he had developed for Zika virus to work for SARS-CoV-2 (COVID-19) which became the “reader” for the devices.
“Basically, you want the ability to do the tests in an efficient manner, and be able to handle up to 10,000 tests per day with high accuracy,” Green said. “This could be used in situations where you want to test students in a particular school district, or where you’re serving the needs of a large community of people, and need to understand the status of the virus inside that population.”
“We have a group of faculty that are very bright, capable individuals, and they really wanted to make a difference,” said Woodbury.
Each of the three efforts will need to pass review and testing by agencies like the U.S. Food and Drug Administration before they can emerge as products we’ll see out in the world, in places like testing labs, medical offices, airports or sports venues. That will probably take at least a year. But from the start, the ASU team was determined that their efforts would go beyond the labs of academia.
“All of these projects had to have a pathway to utilization,” said Woodbury. “In other words, it wasn't, ‘We're going to do some cool stuff and write a paper.’ That was not our objective. We were going to set them each up so that they could be commercialized and move out into the world. And I'm happy to say each of them has a company associated with [them].”
ASU scientists did the research. Three companies are now carrying forward these efforts to commercialization:
- EN CARTA Diagnostics, where Alex Green is a strategic advisor.
- Hayes Lab, led by Mark Hayes.
- Flex Bio Systems and Tech, co-founded by Jennifer Blain Christen.
In the end, these innovative testing devices will be available commercially, applicable not only for COVID-19, but for other infectious diseases as well.
Learn more about better test tech innovations at ASU in this video.


