This week's Lab Notes includes health care and life sciences industry news on a multimillion-dollar stock sale, a new FDA designation and more.
Here's the roundup:
Proscia
The Philadelphia digital pathology services company raised $9 million in a private stock sale, according to documents filed with the Securities and Exchange Commission.
A Proscia spokesman said the raise is an extension of its Series C round, but he declined to identify the investors or disclose how the company plans to use the proceeds.
Proscia was founded in 2014 at Johns Hopkins University by David West, his Hopkins classmate Nathan Buchbinder and Coleman Stavish, a computer expert and West's childhood friend. Proscia moved to Philadelphia in 2017 to be closer to the large volume of young tech professionals coming out of the region's universities.

The company has grown to about 110 employees.
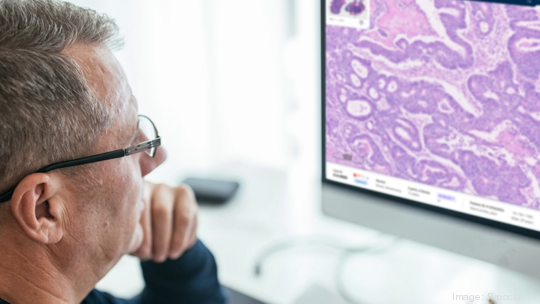
Proscia, which previously raised $75 million through four investment rounds, created a digital pathology platform called Concentriq that features artificial intelligence applications and is used to improve and streamline the analyzing, storing and sharing of digital images of biopsies. The platform was created to automate and standardize processes to accelerate the time it takes pathologists to make a diagnosis.
EpiVario
The Philadelphia biotechnology company, which was spun out of the Epigenetics Institute of the University of Pennsylvania in 2017, has entered into an asset purchase agreement with Metabomed LTD of Israel covering EpiVario's acquisition of a portfolio of compounds with the potential to treat central nervous system diseases and addiction disorders.
Financial terms of the deal were not disclosed.

Thomas Kim, CEO of EpiVario, said the acquisition provides the company with a proprietary, next-generation portfolio of "novel and potent" small molecules known as ACSS2 inhibitors.
EpiVario's cofounders, Dr. Shelley Berger and Dr. Philipp Mews, are leaders in the field of neuroepigenetics, the study of how epigenetic changes to genes — those that can be inherited — affect the nervous system.
Neuraptive Therapeutics
The Wayne biotechnology company received orphan drug designation for NTX-001, its treatment kit targeting peripheral nerve injuries requiring repair.
The designation from the Food and Drug Administration is given to incentivize companies, through tax credits and fee waivers, to develop new drug candidates for rare diseases or conditions. If the treatment is approved, a company gets seven years of market exclusivity.
Evan L. Tzanis, chief operating officer and executive vice president for research and development at Neuraptive, said the company is preparing to meet with regulators in the coming months to seek their input on the future development work and a path to approval for NTX-001.
NTX-001, the company said, is an experimental surgical technology designed to prevent nerve degeneration using an "optimized and specific treatment sequence" in a controlled nerve isolation chamber.
Quick Hits
Radnor-based NRx Pharmaceuticals (NASDAQ: NRXP) said the Nasdaq Stock Market hearings panel granted the company's request for the transfer of its listing to The Nasdaq Capital Market, subject to compliance with all applicable criteria for continued listing on the Capital Market tier including the $35 million in market value of listed securities and the $1 bid price requirements. NRx opened trading on Friday at 42 cents per share and had a market capitalization of $35.8 million. … Rockland Immunochemicals of Limerick launched an impurity detection kit, called the AccuSignal Nucelese Elisa Kit, for the accurate measurement of nuclease across a spectrum of therapeutic manufacturing methods. The kit is designed to be used to evaluate manufacturing processes for biological therapeutics, gene therapy vectors, vaccine preparation and more.