This week's Lab Notes has items on a Wayne drug developer's expanded partnership for a potential rare skin disease treatment, a Malvern medical technology company's expanded regulatory approval overseas and more,
Here's the roundup:
Palvella Therapeutics
The Wayne biopharmaceutical company specializing in treatments for rare genetic skin diseases expanded its partnership with Ligand Pharmaceuticals Inc. to accelerate the development of Qtorin rapamycin, Pavella's experimental treatment for microcystic lymphatic malformations.
Under the terms of the expanded deal, Palvella will receive an upfront payment of $5 million.
In return, Ligand’s existing tiered royalty on worldwide commercial sales of Qtorin increased from 8% to 9.8%. Additionally, Ligand (NASDAQ: LGND) was granted an option to acquire a single-digit royalty on each novel topical product candidate generated from Palvella’s Qtorin platform, which can be exercised at a future date.
The initial target for the therapy, microcystic lymphatic malformations, is a chronically debilitating and lifelong genetic skin disease. There are currently no FDA-approved treatments for the rare condition, characterized by localized masses of malformed lymphatic vessels that protrude through the skin barrier and persistently leak lymph fluid and bleed.
Wes Kaupinen, founder and CEO of Palvella, said Ligand Pharmaceuticals has an established track record in successfully partnering with rare disease companies to accelerate development of "high-value" therapies.
“With an estimated more than 30,000 diagnosed patients in the U.S. suffering from microcystic lymphatic malformations, we see a very significant and attractive commercial opportunity for Qtorin as the first potential FDA-approved therapy for this lifelong disease which causes significant patient morbidity from a very young age," Kaupinen said in a statement.
Palvella previously announced positive phase 2 clinical results from a multi-center study of Qtorin, which is also being studied as a potential treatment for basal cell carcinomas in Gorlin Syndrome.
Aclaris Therapeutics
The Chester County biopharmaceutical company has entered into an exclusive patent license agreement with Sun Pharmaceutical Industries covering the use of deuruxolitinib as a treatment for alopecia.
Under the terms of the agreement, Aclaris (NASDAQ: ACRS), also of Wayne, will receive a $15 million upfront payment from Sun Pharmaceuticals, which is based in India and has its U.S. headquarters in Princeton.
Aclaris will also be eligible for undisclosed regulatory and commercial milestone payments and royalties on any future approved products resulting from the deal.
James Loerop, chief business officer of Aclaris, said the patent license agreement with Sun Pharma is the company's second out-license for the patent portfolio following a deal negotiated last year with Eli Lilly and Co.
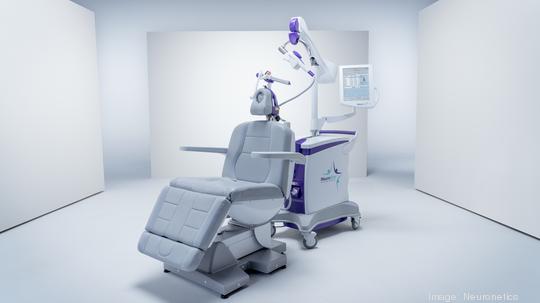
Neuronetics
The Malvern medical technology company has received expanded approval for its NeuroStar TMS (transcranial magnetic stimulation) therapy system from the Pharmaceuticals and Medical Devices Agency in Japan.
Neuronetics (NASDQ: STIM) said Japanese regulators approved multiple proprietary innovations that "significantly advance the patient treatment experience" with NeuroStar for the treatment of major depressive disorder in Japan.
The NeuroStar system, originally approved in Japan in 2017, delivers TMS to the brain using highly focused MRI-strength magnetic field pulses to activate cortical and deep brain structures known to be involved in mood regulation. The treatment can be delivered in a physician's office in under 19 minutes.
An estimated 2.4 million adults live with depression in Japan, according to Neuronetics, with about 655,000 of them being treated by a doctor. The company said of those receiving treatment, an estimated 475,000 patients have failed to achieve remission from depression through antidepressant medications.
To date, more than 5.9 million NeuroStar TMS treatments have been delivered to adults with major depressive disorder.
Quick hits
BriaCell Therapeutics Corp. (NASDAQ: BCTX), which has offices in Philadelphia and Vancouver, announced positive phase 2 study results for its Bria-IMT combination regimen for treating metastatic breast cancer.… Chesterbrook-based Trevena (NASDAQ: TRVN) entered into a purchasing agreement with Premier, under which members of Premier — a national health care improvement organization with more than 4,000 hospital members — will have access to special pricing for Olinyk, Trevena’s acute pain management drug administered in medical centers. Terms of the deal were not disclosed.…Avantia Labs of Ambler raised $1.7 million in an equity financing, according to documents filed with the Securities and Exchange Commission. No further information was immediately available about Avantia.… Berwyn-based Annovis Bio (NYSE: ANVS) appointed Andrew Walsh as its vice president of finance. He was previously senior director of finance and treasury at Ocugen (NASDAQ: OCGN), a biotechnology company based in Malvern.