This week's Lab Notes has details on a new drug launch, a major cash infusion at a biopharm firm, a smaller investment in a medical device company, and more.
Here is the roundup:
Trevena
The Chesterbrook biopharmaceutical company received a $15 million payment from a royalty-based financing deal triggered by the first commercial sale of Olinvyk in China.
The sale was made by Jiangsu Nhwa, the company's licensee in China.
Olinvyk has regulatory approval in the United States and, as of May, in China for adults with acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate.
Trevena (NASDAQ: TRVN) is eligible to receive an additional $10 million under its bridge financing agreement if it achieves certain commercial or financial milestones.
Also this week, Trevena, which is led by CEO Carrie Boudrow, announced positive preliminary data from two Phase 1 proof-of-concept studies of its new drug candidate TRV045.
Boudrow said the company believes TRV045, as a non-opioid therapy, has the potential to make a "meaningful difference" in the lives of patients.
"We look forward to advancing TRV045, on our own or with a strategic partner, [as a] potential treatment of neuropathic pain and other central nervous system disorders,” Boudrow said.
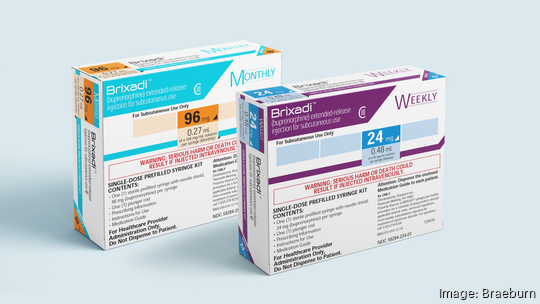
Braeburn Pharmaceuticals
The Plymouth Meeting firm launched its opioid use disorder drug Brixadi in the United States this week, officially concluding a nearly five-year battle to get the injectable medicine into the market.
Brixadi is approved by the Food and Drug Administration as a treatment for moderate to severe opioid use disorder in patients who have initiated treatment with a single dose of a transmucosal buprenorphine product or who are already being treated with buprenorphine. It is available in weekly and monthly doses.
"Opioid use disorder remains a public health crisis with over 82,000 people dying from an opioid overdose last year," said Mike Derkacz, CEO of Braeburn. "We've remained committed to making another option available for health care providers to treat their patients with opioid use disorder and the availability of Brixadi is a significant milestone for Braeburn."
Braeburn received conditional approval for Brixadi from the FDA in late 2018, but delayed the launch of the product because Indivior, a pharmaceutical company with offices in the United Kingdom and Virginia, was previously granted orphan drug designation for a competing product, called Sublocade, that gave it market exclusivity.

Braeburn filed a citizen petition in April 2019 with the FDA, asking for the federal government to revoke Indivior's orphan designation for Sublocade, arguing opioid use disorder is not a bona fide orphan disease. Such a designation is indicative of conditions affecting 200,000 or fewer people.
The FDA granted Braeburn's citizens petition in November 2019.
Braeburn then had to resubmit its new drug application for Brixadi in 2021, after the FDA failed to provide final approval of the new drug candidate in December 2020 because of deficiencies found during an inspection at a third-party manufacturing facility. Braeburn responded to additional questions from the FDA about the application last year, which led to Brixadi's final approval in May.
Runway Healthcare
Pace Medical, a Malvern medical device company, raised $400,000 in an equity financing.
The Chester County company was founded earlier this year by Runway Healthcare, a Chester County medtech accelerator led by Jeffrey F. O'Donnell Sr., Runway's managing general partner.
Pace, which is focused on developing products for use by orthopedic surgeons in foot and ankle repair, disclosed the private stock sale in documents the company filed with the Securities and Exchange Commission.
Runway previously launched two other Malvern companies: Waypoint Orthopedics and Toetal Solutions.
Waypoint this week announced orthopedic spine surgeon Dr. Stephen Banco completed the first-in-man spine surgery using the Waypoint GPS smart pedicle probe designed to provide visualization technology with standard instrumentation.
"This technology has the capacity to redefine how we approach spine surgeries, Banco said in a statement provided by Waypoint. "Its accuracy and real-time feedback empower surgeons to make informed decisions during procedures, resulting in better patient outcomes and reduced recovery times."
Quick hits
Verismo Therapeutics of Philadelphia said the first patient was dosed in a Phase 1 clinical trial of its experimental cancer therapy SynKIR-100. The company is developing the new drug candidate as a potential treatment for advanced ovarian cancer, mesothelioma, and cholangiocarcinoma. … Plymouth Meeting-based Inovio (NASDAQ: INO) was granted breakthrough therapy designation for INO-3107, its experimental treatment for patients with recurrent respiratory papillomatosis, from the FDA. The designation, the first for an Inovio DNA medicine candidate, is designed to expedite the development and review of drug candidates that are intended to treat a serious or life-threatening condition. … The FDA granted orphan drug designation to pitolisant, which Harmony Biosciences (NASDAQ: HRMY) of Plymouth Meeting is developing to treat a daytime sleeping disorder known as idiopathic hypersomnia. The designation provides drug companies with benefits such as tax credits and user fee exemptions as an incentive to develop new medicines to treat rare conditions.