
This week's life sciences and health care news includes a cell therapy company merger, a Big Pharma initiative aimed at boosting vaccine rates, progress reports on acne and epilepsy drugs, and more.
Here's the roundup:
Adaptimmune
The cell therapy developer with operations in Philadelphia and Oxfordshire, England, completed its previously announced acquisition of TCR² Therapeutics.
Under the terms of the all-stock deal valued at about $110 million, Adaptimmune (NASDAQ: ADAP) shareholders own about 75% of the combined company and TCR²’s previous stockholders own the remaining 25%.
“Together, we will advance our industry leading pipeline making cell therapy a mainstream option for people with cancer,” said Adrian Rawcliffe, CEO of Adaptimmune.
Rawcliffe said the combine company’s initial goals include gaining approval for its engineered T-cell therapy for a solid tumor, afami-cel, an experimental treatment for a type of soft tissue cancer known as synovial sarcoma. He said the company is also progressing its mid-stage clinical testing of a potential ovarian cancer therapy.
The combined company, led by Rawcliffe, is trading on the Nasdaq Stock Market under the symbol "ADAP." Its board of directors is now composed of three members from TCR² of Cambridge, Massachusetts, and six continuing from Adaptimmune.
GSK
The London-based Big Pharma company with operations in Philadelphia and Montgomery County, launched what it is calling the COiMMUNITY Initiative to help reduce health inequities and “set a new precedent” for adult immunization rates in the United Rates, which GSK (NYSE: GSK) said continue to remain below pre-pandemic levels.
Under the initiative, GSK is committing up to $1 million in grant funding to support national, state and local nonprofit organizations and community-based groups focused on adult immunization and health equity. The company will also work to strengthen data transparency and access to immunization trends to inform public health efforts, which will include enhancing its Vaccine Track platform and opening it to public and private stakeholders. GSK said it will also share new resources, tools and best practices in adult vaccine confidence and delivery to help organizations implement “tangible solutions” for addressing adult immunization gaps.

"This is a critical moment for public health,” said Judy Stewart, senior vice president and head of vaccines at GSK. "While there is a heightened level of awareness of the importance of vaccines to prevent infectious diseases, too often, adults miss the opportunity to prioritize vaccination, despite being increasingly susceptible to immune decline and infectious diseases. Our industry can do more by investing in local interventions to help create healthier communities and sustain best practices from the pandemic. We believe this initiative will contribute to a more equitable and resilient public health infrastructure and bolster existing partner efforts — leading to more vaccinated adults."
Strata Skin Sciences
The Horsham-based medical technology company said the first six patients have been treated in a study measuring the effectiveness of TheraClearX as a treatment for mild to moderate acne.
The purpose of the study, according to Strata (NASDAQ: SSKN), is to further substantiate the efficacy and safety of TheraClearX as a standalone acne treatment for healthy teenagers and young adults.
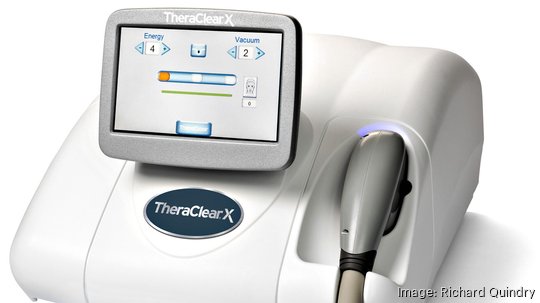
TheraClearX is designed to treat acne by combining vacuum technology and intense broadband light. The Food and Drug Administration-approved device was launched last year by Strata, which acquired the technology from California-based Theravent Corp. at the start of 2022.
The Strata study is being led by Dr. Glynis Ablon and conducted at the Ablon Skin Institute and Research Center in Manhattan Beach, California. The treatment will be tested in 30 healthy male and female subjects over the course of seven weeks.
“TheraClearX has the potential to make a meaningful impact on those who suffer from mild to moderate acne through our vacuum technology and intense broadband light that targets the sebaceous glands,” said Bob Moccia, Strata’s CEO. “We expect patients that have been seen in the clinic to experience visible reductions in acne lesions.”
Acne is the most common skin disease in the United States affecting up to 50 million people, according to the American Academy of Dermatology Association. An estimated $5.5 billion is spent annually on acne treatments.
Marinus Pharmaceuticals
The Radnor pharmaceutical firm said the Committee for Medicinal Products for Human Use of the European Medicines Agency has adopted a positive opinion recommending the approval of Ztalmy for the adjunctive treatment of epileptic seizures associated with cyclin-dependent kinase-like 5 deficiency disorder (CDD) in patients 2 to 17 years of age.
A final decision on Ztalmy by the European Commission is expected by early August and, if approved, be applicable to all 27 European Union member states plus Iceland, Norway and Liechtenstein. If approved, Ztalmy will be the first treatment in the European Union for the treatment of seizures associated with CDD. It will be commercialized by Orion Corp.
“This recommendation brings us one step closer to addressing a significant unmet need for CDD patients with treatment-resistant seizures in Europe," said Dr. Scott Braunstein, CEO of Marinus (NASDAQ: MRNS).
Ztalmy is already approved in United States by the FDA for the treatment of seizures associated with CDKL5 deficiency disorder in patients 2 years of age and older.
Marinus is also studying the therapy in other rare seizure disorders, including tuberous sclerosis complex and refractory status epilepticus.
Quick hits
Chesterbrook-based Trevena (NASDAQ: TRVN) recently announced it has received notice from Nasdaq that the biopharmaceutical company has regained compliance with the exchange's $1 per share minimum bid price requirement. … The FDA accepted for review a biologics license application filed by Iovance Biotherapeutics (NASDAQ: IOVA) for lifileucel, its lead cell therapy candidate targeting melanoma. A decision on the experimental skin cancer treatment is expected by Nov 25. Iovance is based in California and has a cell therapy manufacturing campus at the Philadelphia Navy Yard.







