
This week's Lab Notes has items on a pending merger proposal, a medical device company's multimillion-dollar debt financing deal, a $10 million gift that will support research for a genetic disorder and more.
Here is the roundup:
Children's Hospital of Philadelphia
The Philadelphia pediatric care provider received more than $10 million in a series of gifts from the Gilbert Family Foundation to fund critical neurofibromatosis research.
Dan and Jennifer Gilbert established their private foundation in 2015 to accelerate a cure for neurofibromatosis type 1, or NF1, a genetic disorder that can affect multiple systems of the body. Nick Gilbert, the couple's oldest son, was diagnosed with NF1 as an infant and continues to battle the disease. The condition is characterized by symptoms that include skin changes, skeletal abnormalities, and tumors that develop in the brain and body and can lead to serious complications including vision loss and severe pain.
The gift will fund a CHOP-led, multi-institutional research project aimed at developing evidence-based or “standard of care” guidelines for the management of children with NF1-associated brain tumors occurring outside of the visual pathway. The research studies could also lead to the discovery of immunotherapy targets for the disease.
“These grants from the Gilbert Family Foundation will allow us to conduct crucial studies that we hope will eventually lead to better treatments for those with NF1,” said Dr. Michael Fisher, director of the neurofibromatosis program at CHOP.
Neurofibromatosis type 1, according to CHOP, occurs in about one in 3,000 people — with about half of the cases occurring sporadically and the other half being hereditary. The genes causing the disorder may be passed from generation to generation in a family, however, the type and severity of the disease can vary widely among affected family members.
Vallon Pharmaceuticals
The Philadelphia biopharmaceutical company said more than 90% of its shareholders have voted in support of its proposed merger with GRI Bio Inc. of San Diego.
Vallon (NASDAQ: VLON), however, said additional votes are needed in order to close the deal, first announced in December.
"We appreciate the strong support from Vallon’s voting stockholders and strongly urge all unvoted stockholders of record to vote for all proposals in order to move forward with the merger," said David Baker, the company's CEO, in a statement. "Both Vallon and GRI Bio are excited about the compelling opportunities that the merger brings to their respective stockholders.”
The company noted that among the five proposals shareholders are being asked to vote on, one of particular importance is the approval of a reverse stock split that carries a higher vote threshold than some of the other proposals. The merger cannot be consummated unless the reverse stock split is approved.
A special stockholder meeting is scheduled to be held virtually on April 12.
California-based GRI Bio is developing what are known as Natural Killer T cell regulators to treat inflammatory, fibrotic and autoimmune disorders.
Founded in 2018, Vallon has spent the past eight months evaluating “strategic alternatives" after the company's lead new drug candidate, an experimental treatment for attention deficit hyperactivity disorder, failed in clinical testing.
Vallon said the merger will result in a clinical-stage biotechnology company "focused on discovering, developing, and commercializing innovative therapies targeting serious diseases associated with dysregulated immune responses that lead to inflammatory, fibrotic, and autoimmune disorders."
Join us for Gene Editing: Where are we, where are we going on June 22
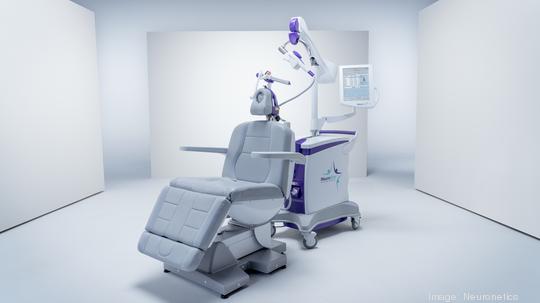
Neuronetics
The Malvern medical technology company has secured up to $60 million through a new debt financing agreement with SLR Capital Partners.
Keith J. Sullivan, president and CEO of Neuronetics (NASDAQ: STIM), said the incremental, non-dilutive debt financing will further strengthen the company's balance sheet and allow it to continue to seek to expand the commercial adoption of its NeuroStar Advanced Therapy for mental health.
Neuronetics' NeuroStar system provides a non-drug and noninvasive treatment for neurohealth conditions, such as major depressive disorder, that involves the use of transcranial magnetic stimulation. The system delivers highly focused MRI-strength magnetic field pulses to activate cortical and deep brain structures known to be involved in mood regulation. The treatment can be delivered in a physician's office in under 19 minutes.
Neuronetics also said this week it has converted about $5.9 million in an outstanding accounts payable balance owed to the company by Greenbrook TMS, a treatment center operator and Neuronetics partner, into a $6 million promissory note.
Sullivan said the company's goal continues to be "achieving cash flow breakeven" in 2024.
Last year, the company generated revenue of $65.2 million while posting a net loss of $37.2 million.
Quick Hits
Sparing Vision, a French genomic medicine company with U.S. operations in Philadelphia, named former Bausch and Lomb Chairman and CEO Joseph C. Papa as its non-executive independent chairman. Sparing Vision's lead drug candidate, SPVN06, is entering clinical testing as a potential gene therapy for a retinal disease known as retinitis pigmentosa. … Philadelphia-based Verismo Therapeutics received Fast Track Designation from the FDA for SynKIR-110, the University of Pennsylvania spinout company's experimental therapy for treating mesothelioma, a type of cancer associated with asbestos exposure. … YPrime, a Malvern-based clinical trial software products and services company, named Jim Corrigan as its new CEO. Corrigan, previously CEO of ERT and ConnectiveRx, succeeds Shawn Blackburn who had served as CEO of the company since he founded it in 2006. Blackburn will remain with the company as the leader of a new team called YPrime Labs, which is focusing on future technology to enhance the experience of patients in drug studies.







