This week's life sciences industry news includes details on a medical device company's legal settlement, a CEO change at one local firm and a management team expansion at another, a new product launch, and more.
Here's the roundup:
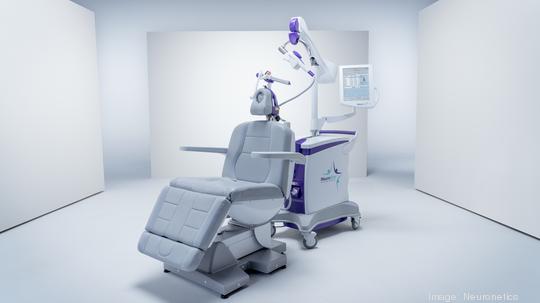
Neuronetics
The Malvern medical technology company settled its unfair competition litigation against competitor Brainsway Ltd. and Brainsway USA Inc.
Last year, Neuronetics (NASDAQ: STIM) — developer of the NeuroStar system, a noninvasive treatment for patients suffering from major depressive disorder and obsessive compulsive disorder — filed a lawsuit at U.S. District Court against Brainsway. The lawsuit alleged Massachusetts-based Brainsway, which markets a competing product, "egregiously misrepresented" efficacy data for the treatment of anxious depression with the NeuroStar system to mislead clinicians and patients.
The NeuroStar system delivers transcranial magnetic stimulation (TMS) to the brain, using highly focused MRI-strength magnetic field pulses to activate cortical and deep brain structures known to be involved in mood regulation. The TMS treatment can be delivered in a physician's office in under 19 minutes.
Under the terms of the settlement, BrainsWay agreed to stop the use of the efficacy data that was at issue in the complaint filed by Neuronetics.
“As I said at the outset of this litigation, Neuronetics will vigorously defend our data and the efficacy of NeuroStar,” said Keith J. Sullivan, CEO of Neuronetics, in a statement. "The parties have agreed to keep the additional terms of the settlement agreement confidential."
Christopher von Jako, CEO of BrainsWay, said his company is "pleased to have resolved this litigation so that we can focus our full attention on our technology and bringing our Deep TMS therapy to patients."

Harmony Biosciences
The Plymouth Meeting company announced John J. Jacobs has stepped down as the company’s CEO to pursue other opportunities.
Dr. Jeffrey Dayno, Harmony’s executive vice president and chief medical officer, was named interim CEO. Jeff Aronin, the company’s founder and board chairman, was named executive chairman.
"We have full confidence in Dr. Dayno taking over as CEO on an interim basis," Aronin said. "He has been instrumental in leading our clinical development programs and regulatory strategy as our chief medical officer for the past five years. He is a nationally recognized expert in neuroscience and has been responsible for managing the research, development and medical and regulatory affairs for Harmony.’

Founded in 2017, Harmony (NASDAQ: HRMY) markets the narcolepsy drug Wakix, which was approved by the Food and Drug Administration in 2019. Wakix sales propelled the company's net income to $132.8 million for the first nine months of 2022, compared to $11.9 million during the same period in 2021.
Harmony went public in 2020 raising $128.4 million through its initial public offering, which was priced at $24 per share. The company’s stock opened Friday at $47.71 per share.
Annovis Bio
The Berwyn biotechnology firm developing treatments for Alzheimer’s and Parkinson’s diseases added two members to its management team.
Michael Christie will serve as vice president of process chemistry at Annovis. David Prohaska was named vice president of toxicology and pharmacology.
Maria L. Maccecchini, the founder and CEO of Annovis (NYSE: ANVS), said Christie and Prohaska will “significantly strengthen the company’s ability to conduct successful clinical trials and navigate the regulatory process.”

Christie, who has more than 40 years of experience in the pharmaceutical industry, was previously senior director of chemical process research and development for Teva. He held the same job at Cephalon, which was acquired by Teva in 2011. Prohaska has 25 years of experience in preclinical drug development. He was previously at Aravive Biologics, where he served as the director of preclinical development and clinical operations support.
Tela Bio
The Malvern medical technology company this week launched its NIVIS Fibrillar Collagen Pack.
The pack is an absorbent matrix of type I and type III bovine collagen designed to manage moderately to heavily leaking wounds and to control minor bleeding.
Type I and type III collagen, according to Tela, closely resemble the native collagen of a patient and have been shown to stimulate cellular activity and contribute to new tissue development.

Antony Koblish, CEO of Tela Bio, said the NIVIS product "perfectly" complements his company's OviTex portfolio and provides it with a new "growth vehicle."
OviTex is Tela's reinforced tissue implants that combine biologic and synthetic materials and are designed for hernia and abdominal wall reconstruction. NIVIS is provided in particulate form so it can be molded and packed into wounds.
Tela Bio (NASDAQ: TELA) last April entered into an exclusive development and distribution partnership for Collagen Matrix Inc.'s proprietary fibrillar collagen pack in the United States.
Quick Hits
AstraZeneca (NYSE: AZN) received FDA approval for Airsupra, a rescue medication for as-needed use to prevent bronchoconstriction and reduce the risk of asthma exacerbations in adults. The treatment was developed by AstraZeneca in a partnership with Avillion. … Ocugen (NASDAQ: OCGN) of Malvern announced positive late-stage study results for Covaxin, the Covid-19 vaccine it is working to bring to the United States in a partnership with Bharat Bio of India. Covaxin demonstrated the ability to generate a broader immune response against the whole virus, covering important antigens such as S-protein, RBD, and N-protein. Ocugen noted currently approved vaccines in the U.S. target only S-protein antigen. Additionally, the study showed Covaxin induces robust long-term memory B- and T-cell responses. Dr. Shankar Musunuri, Ocugen's CEO, said the study data will be critical to support Ocugen’s future plans for seeking approval for Covaxin from the FDA. … Radnor-based Avantor Performance Materials (NYSE: AVTR) entered into a multiyear supply and services agreement with Catalent Inc. (NYSE: CTLT) of Somerset, New Jersey. Under the terms of the agreement, Avantor will be the primary supplier of a broad range of laboratory supplies, clinical and production materials, and services to Catalent, expanding the companies' existing relationship. Specific financial terms of the deal were not disclosed.







