
This week's life sciences industry news includes details on a new development in field compounded medications for animals, a medical device approval, a CFO hire, a multimillion-dollar stock sale, and more.
Here's the roundup:
Wedgewood Pharmacy
The South Jersey compounding pharmacy, which focuses exclusively on animal health, conducted a nationwide poll of veterinary practices that found 69% are not confident they are prepared to comply with new regulations from the Food & Drug Administration for compounding animal drugs from bulk drug substances.
The new regulations were finalized on April 13 and are slated to be enforced beginning Oct. 1.
Wedgewood received responses to its survey from 1,574 veterinary professionals across the country, all of whom work in practices that prescribe compounded medications for animal health. The company said it wanted to learn how much its customers knew about the FDA’s new guidance and the changes it will require to ordering and prescribing procedures.
"We are working in a very focused way across our organization and industry not only to comply with the letter and spirit of this guidance, but to help our more than 60,000 veterinary prescribers to interact with us so that they, too, are in compliance when they order compounded medications," said Marcy A. Bliss, CEO of Wedgewood Pharmacy.
Bliss said the FDA's new guidance will change the way medication is obtained by veterinary practices for difficult-to-treat patients, specifically animals whose needs cannot be met by FDA-approved alternatives.
Meghann Abbott, assistant product manager for Wedgewood Pharmacy, said the Swedesboro company is taking an "aggressive lead" in nominating bulk drug substances to FDA for approval for office-use compounding.
"So far, we have nominated more than 50 families of medication," Abbott said. "We have devoted significant resources to this nomination process because we strongly believe that veterinarians should be able to stock the medications they believe are critical to immediate patient care.”
Neuronetics
The Malvern medical technology company's D-Tect MT Accessory received marketing clearance from the FDA.
The D-Tect device was designed to make it easier for physicians to determine a patient's motor threshold, a means of quantifying stimulus in transcranial magnetic stimulation (TMS).
Cory Anderson, vice president of research and development at Neuronetics (NASDAQ: STIM), called the FDA clearance "the next chapter in our plan to simplify and accelerate the [motor threshold] determination for NeuroStar providers."
Neuronetics' Neurostar system delivers highly focused MRI-strength magnetic field pulses to activate cortical and deep brain structures known to be involved in mood regulation. The TMS treatment can be delivered in a physician's office in under 19 minutes.
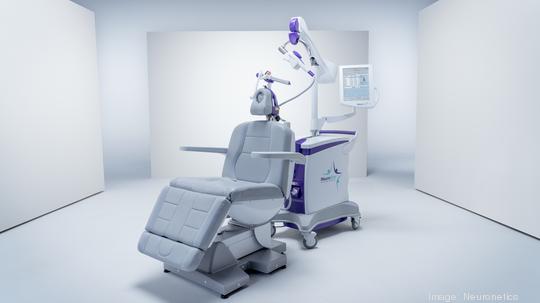
The D-Tect device aids clinicians by visually reporting the magnitude of finger movements during motor threshold mapping of patients being treated for major depressive disorder. D-Tect is designed to allow the motor threshold determination to be performed by only one person. It does not require cords or sensors to be attached to the patient.
The D-Tect MT Accessory, the company said, will initially be available via a limited commercial launch. A national rollout is set to begin in mid-September.
Annovis Bio
The Berwyn biopharmaceutical company developing treatments for Alzheimer's and Parkinson's diseases has hired Henry Hagopian III as its new chief financial officer, effective immediately.
Hagopian has 30 years of finance and accounting experience, including 15 years at Organogenesis, a publicly traded regenerative medicine company.
Annovis Bio (NYSE: ANVS) said its former CFO, Jeff McGroarty, "has stepped away from his role to pursue other interests." The company said McGroarty will assist the company to provide an orderly transition of his duties to Hagopian over the next several weeks.
Maria L. Maccecchini, founder and CEO of Annovis, called Hagopian "an excellent addition to our team [who] will help take the company to the next level given his extensive experience in finance, accounting and strategic execution."
Baudax Bio
The Malvern pharmaceutical company completed its previously disclosed $6.2 million public stock sale.
Baudax Bio (NASDAQ: BXRX) sold slightly more than 11.8 million shares of its common stock, together with accompanying common stock purchase warrants, for 52.5 cents per share. The warrants have an exercise price of the same 52.5 cents per share.
The company plans to use the proceeds from the stock sale for "pipeline development activities" and general corporate purposes such as research and development costs, manufacturing costs, and working capital.
Baudax Bio has one product, Anjeso, in the market. Anjeso is a non-opioid and non-steroidal anti-inflammatory drug approved for the management of moderate to severe pain. The intravenous drug, used in hospitals and other acute care settings, received marketing clearance from the FDA in early 2020. The company's pipeline includes two clinical-stage neuromuscular blocking agents, which are drugs used for general anesthesia, and a proprietary chemical reversal agent specific to its experimental blocking agents.
H.C. Wainwright & Co. served as the exclusive placement agent for the offering.
Quick Hits
Radnor-based Marinus Pharmaceuticals (NASDAQ: MRNS) finalized the sale of its rare pediatric disease priority review voucher to Novo Nordisk for $110 million. Marinus plans to use the proceeds to support its commercial launch of Ztalmy, advance its new drug pipeline and for other general expenditures. … Lannett Co. (NYSE: LCI) of Trevose completed subject dosing in the pivotal clinical trial of its biosimilar insulin glargine, a product the company is co-developing with its strategic alliance partners within the HEC Group of companies. Study results are expected later this year. … The Plymouth Meeting-based National Comprehensive Cancer Network has entered into a collaboration with Pfizer (NYSE: PFE) and Myovant Sciences to fund research projects seeking to improve cardiovascular management of patients with prostate cancer who are being treated with androgen-deprivation therapy.







