This week in the Philadelphia region's life sciences industry saw a multimillion-dollar acquisition, an FDA approval, a potential Covid-19 product update and two orphan drug designations. Here's the rundown:
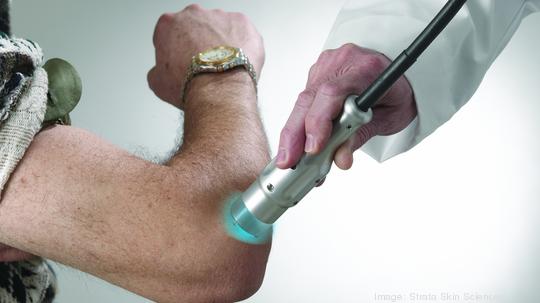
Strata Skin Sciences
The Horsham-based medical device company acquired the U.S. dermatology business of Ra Medical for an upfront cash payment of about $3.7 million plus the assumption of customer warranty and service agreement liabilities. Strata Skin Sciences (NASDAQ: SSKN) said the acquisition positions the company as "the predominant provider" of excimer laser treatments designed to address the $6 billion U.S. market for the treatment of chronic skin diseases. Strata will now market both its Xtrac excimer laser system and California-based Ra Medical's Pharos excimer laser system to treat a variety of chronic skin diseases, including eczema, psoriasis, vitiligo, atopic dermatitis and leukoderma. Robert J. Moccia, Strata's CEO, said the transaction provides the company with an opportunity to increase its current installed laser system base, which now encompasses more the 850 dermatology partners, by more than 40%.
Virpax Pharmaceuticals
The Berwyn drug developer said it received a written pre-investigational new drug response from the Food and Drug Administration for MMS019, its patented and proprietary high-density molecular masking spray under development for use as an anti-viral barrier product. Anthony P. Mack, CEO of Virpax, said the company believes the results of the pre-IND response from the FDA support further research on MMS019 as an intranasal protective that may limit transmission of viruses including Covid-19. Virpax expects to pursue a new drug application for MMS019 as a once-daily, over-the counter intranasal treatment. The company is working with Syneos Health on a clinical trial design and timeline.

Globus Medical
The Audubon medical device company was granted marketing clearance for its Excelsius3D imaging system by the Food and Drug Administration. The Excelsius3D platform consolidates three types of imaging technology — cone beam computed tomography, fluoroscopy, and high-resolution digital radiography — in one unified device. The product is designed to eliminate the need for multiple imaging systems during one procedure. Globus Medical (NYSE: GMED) representatives said the company is ramping up production and preparing for commercial release of the mobile X-ray system in the fourth quarter of this year.
Orphan drug designations
Two life sciences companies with ties to the region received orphan drug designation for new drug candidates. The designation — which comes with tax credits, exemptions from certain federal application fees, and potential market exclusivity — is granted by the Food and Drug Administration as an incentive for companies to develop treatments for diseases or conditions affecting fewer than 200,000 people in the United States.
Wayne-based NFlection Therapeutics was granted orphan drug designation for NFX-179, an experimental treatment for cutaneous neurofibromatosis type 1. Cutaneous neurofibromas are tumors that grow from small nerves in the skin, or just under the skin, and appear as small or larger bumps typically beginning around the time of puberty. NFX-179 is in mid-stage clinical testing.
Boston-based Paratek Pharmaceuticals (NASDAQ: PRTK), which has a large presence in King of Prussia, received an orphan drug designation for Nuzyra, an experimental treatment of infections and pulmonary disease caused by nontuberculous mycobacteria. Nuzyra is in mid-stage clinical testing.







