A Minnesota-based medical device company with Wisconsin ties recently took a big step toward commercialization.
HistoSonics Inc. earlier this month received approval from the Food and Drug Administration to market its histotripsy platform — a device that destroys cancerous tissue with sound waves.
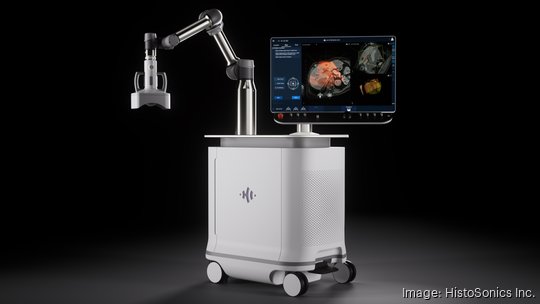
The company is now moving ahead quickly with the commercialization of its Edison medical device, which is aimed at treating liver tumors without using a scalpel or needles.
Multiple Wisconsin investors and researchers have been involved with HistoSonics' path to commercialization.
Venture Investors LLC — a venture capital firm with locations in Madison, Milwaukee and Ann Arbor, Michigan — was an early investor in HistoSonics. It led the company's 2009 spin-out from the University of Michigan and also led the startup's first two rounds of financing, according to Venture Investors chief financial officer David Arnstein.
Additionally, Venture Investors chief medical and scientific officer Joe Amaral serves as vice president of medical affairs for HistoSonics, and Venture Investors executive managing director Jim Adox chairs the HistoSonics board.

Commercialization ahead
For the first time, hospitals can begin to acquire HistoSonics' Edison system in routine clinical care instead of just as part of a more restrictive clinical trial. Edison was approved for liver tumors, and HistoSonics has now begun a kidney tumor trial and plans to begin a pancreatic tumor trial early next year.
“It’s an amazing milestone," HistoSonics president and CEO Mike Blue said. "Nothing that is this novel and new is easy when it comes to clinical trials and regulatory clearances.”
The University of Wisconsin-Madison played a critical role in animal studies prior to the clinical trial, and the Medical College of Wisconsin treated a patient as an approved site in the clinical trial, Arnstein said in an email.
The market authorization happened through the FDA’s De Novo Classification Request process, a review pathway for medical devices that have no existing predicate. Edison is the first and only histotripsy platform available in the U.S., according to HistoSonics.
HistoSonics began receiving purchase orders from hospitals across the country immediately after the FDA offered De Novo classification, HistoSonics vice president of marketing Josh King told Wisconsin Inno's sister publication in Minnesota.
The company has grown to 110 employees, most of whom are at its headquarters in Plymouth, Minnesota. It raised $100 million last year and has raised a total of nearly $227 million across 10 funding rounds.







