When MBJ first spoke to Mark Myers in February 2022, he said the “ever-living hamster wheel” of work had taken up most of his weekend.
Roughly 11 months later, he doesn’t seem to have gotten off that wheel.
Because Myers is still an assistant professor of anatomy and neurobiology at the University of Tennessee Health Science Center (UTHSC). He’s still an adjunct professor at Christian Brothers University (CBU). He’s still a project management consultant for FedEx. And he’s still the leader of NeuroDyne, the Memphis-based medical device startup that’s hoping to make the epilepsy diagnosis process considerably easier for patients.
The constant stream of activity, however, hasn’t prevented Myers’ fledgling company from finding success. NeuroDyne made significant strides last year, and recently, MBJ caught up with Myers to chat about its latest developments.
Here are the highlights.
NeuroDyne’s primary goal
Before we dive into its recent work, let’s recap exactly what NeuroDyne is hoping to accomplish.
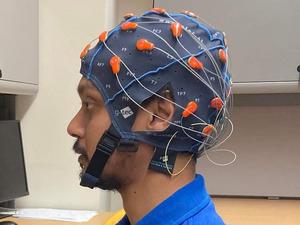
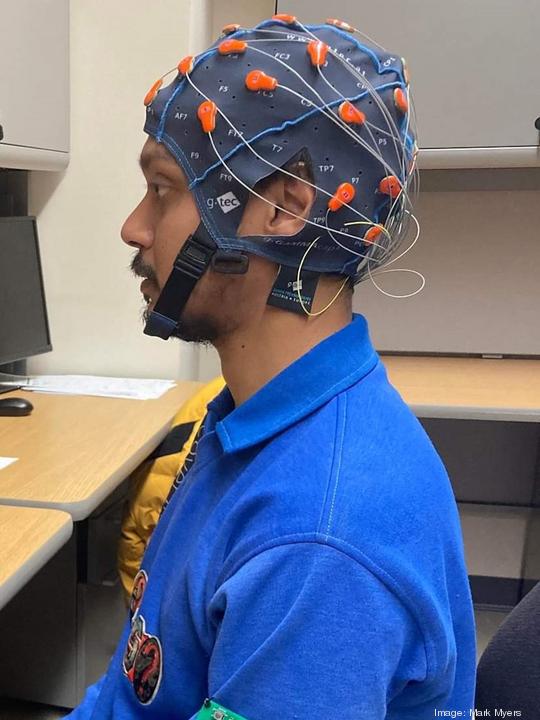
The startup has developed a portable cap that collects data for an epilepsy diagnosis, all from the patient’s home. But why is this significant?
Often, data must be collected in a hospital’s epilepsy monitoring unit, where electrodes are attached to the patient’s scalp through an electroencephalogram (EEG), which records the electrical activity of the brain. But, in many cases, these electrodes are attached to the scalp with a gel that is difficult to remove and a bundle of wires hooked up to a computer system. You can’t move around much during the testing — even going to the bathroom can be a big deal.
And the diagnosis process isn’t short: It can take three to seven days, and the costs add up quickly for the patient, insurance, and health provider. The stay doesn’t necessarily lead to a perfect diagnosis, either. Even data from a weeklong stay might only show one or two seizure instances, which the doctor must use to determine the type of seizure in question and make a diagnosis.
Myers believes that his startup’s device could fix many of these problems.
Because the patients will be wearing the cap at home rather than during an in-patient stay at the hospital, the cost of diagnosis could be cut drastically. The NeuroDyne cap doesn’t require the gel, with the electrodes instead being connected with a set of four prongs that are rubbed against the scalp. It also allows for significantly more mobility — and its latest iteration has been notably improved.
NeuroDyne new algorithm and connection
Previously, NeuroDyne’s cap had a Wi-Fi transmitter that sent EEG signals to a tablet. This tablet had a Wi-Fi receiver and was installed with NeuroDyne’s app, which processed the incoming signals.
But, as Myers said, brain activity produces “tiny, tiny" signals. And those biosignals have to compete with others that can stem from even simple body movements and muddle their detection by an EEG.
“Any time someone moves an arm or moves their head, they [produce] signals that actually compete with regular biological signals from the head,” Myers explained. “The need to remove those type of artifacts is huge.”
So, his startup has developed an algorithm that will remove extraneous signals coming from things like hand and head movement, and “get right to the heart of the brain signals” — which are needed for the epilepsy diagnosis.
“Because we’re mobile, that’s a big deal,” Myers said.
While NeuroDyne has added an algorithm to the device, it’s removed the Wi-Fi component. Rather than wirelessly connecting to the tablet, the cap now hooks up to it directly; Wi-Fi is no longer needed. The new version is a cellular device that's more efficient and has a noticeably longer battery life.
“Now we can do some degree of power optimization,” Myers said. “Because so much of the power had gone into the Wi-Fi.”
Zeroto510, and NeuroDyne funding
The device improvements stemmed, in part, from Myers’ participation in the 2022 ZeroTo510 Medical Device Accelerator, Epicenter’s program that helps early-stage medical device startups refine their business model and navigate through the startup process.
The accelerator opened new doors for NeuroDyne. It provided the startup with further mentorship and access to reimbursement experts and manufacturers — which it will need in the future.
“The ZeroTo510 acceptance was huge for us,” Myers said. “There are other accelerators, but this group was really well known for their breadth of expertise.”
NeuroDyne has also attracted interest from Semmes Murphey Clinic, which focuses on brain and spine care; and it’s applying for a Phase Two SBIR grant from the National Science Foundation (NSF) that’s valued at $1 million.
If it scores this federal grant, the entrepreneurial support organization LaunchTN is expected to provide another $300,000 — bringing the total haul to $1.3 million. And that could go a long way toward NeuroDyne’s current goal of raising $3.4 million. Currently, NeuroDyne has around $425,000 in seed funding.
The additional funds are slated to help NeuroDyne pay for a variety of necessary tasks, including manufacturing, product optimization, and sales development. They’re also set to help the startup get through the costly process of gaining FDA approval.
Right now, the goal is for NeuroDyne to gain FDA approval in about 18 months, and to do this, it will need to test its device on additional patients, a process that isn't cheap.
“The feedback we received is, ‘Test another 35 to 40 patients,’” Myers said. “And we could achieve Class I FDA approval, which gets us to market."