The good manufacturing practice (GMP) facility at St. Jude Children's Research Hospital has new leadership, the organization announced on Wednesday, July 27.
Frank Fazio has taken over as director of the hospital's GMP facility, which is dedicated to producing specialized biopharmaceuticals for the hospital's clinics. He will serve as the president of Children's GMP LLC, the St. Jude-owned operating unit providing manufacturing services at the facility, and as VP of therapeutics production and quality.
"I am excited to lead the talented team at the GMP, as well as therapeutics production and quality, to continue to translate and manufacture high-quality, novel, and advanced therapeutics across a broad range of modalities," he said in a press release.
The lifesaving mission of St. Jude is a big draw for many new hires, and Fazio, too, said he viewed his new role as “an incredible opportunity” to contribute to that mission.
Fazio has spent much of his career working in GMP manufacturing and management. Before coming to Memphis, he served as the deputy executive vice chancellor of MassBiologics, the GMP manufacturing segment of the University of Massachusetts Chan Medical School. According to his LinkedIn profile, Fazio had been at MassBiologics for nearly 13 years.
He has also worked with a host of pharmaceutical firms like Pfizer, Boston Scientific, and Becton, Dickinson & Co., and holds an MBA from Anna Maria College, along with an M.S. in work environment from the University of Massachusetts Lowell.
Fazio's grasp of organizational and foundational knowledge, combined with his management skills, approachability, and the experience he brings — having worked with different players in designing and manufacturing advanced therapeutics — is what impressed the St. Jude faculty and staff, Dr. Terrence Geiger, Ph.D., SVP and deputy director for academic and biomedical operations at St. Jude, said in the release.
"Individuals with significant experience in leading high-end academic GMP manufacturing are few, and those willing to move are truly rare,” Geiger said. “In such circumstances, we are fortunate to be in an institution with a mission, direction, and people that are attractive to the best talent.”
Fazio takes over as director of the St. Jude GMP facility from Michael Meagher, Ph.D., who served in the role for over a decade before retiring in June. In the release, Geiger expressed his gratitude toward Meagher for the latter’s work in building the manufacturing facility's capabilities into what they are today.
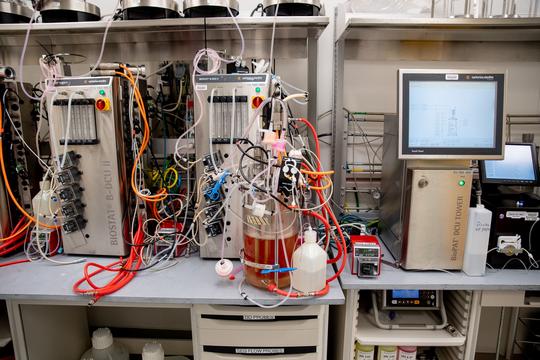
Tour of St. Jude's GMP facility
Launched in 2003, the 64,000 square feet St. Jude GMP facility sits at the northeast corner of Third Street and Shadyac Avenue. The facility is crucial in manufacturing biopharmaceutical products and other innovative treatments for patients per federal guidelines and moving these discoveries from St. Jude labs into its clinic. It manufactures various products, including live virus vaccines, gene therapy products, cellular therapy products, monoclonal antibodies, and recombinant proteins.
The facility has two components- the TPQ, where the St. Jude TPQ staff defines the analytical methods required to assist in the production of biopharmaceutical products and provides the process development and scale-up services, and the Children's GMP, LLC, which provides the manufacturing services for the products. The facility allows St. Jude to fast-track the development and production of innovative treatment options that big pharmaceutical companies may not initially want to invest in. According to the facility's website, royalties earned from these products are reinvested into research to help continue developing cures for childhood cancer.