When Idicula Mathew started analyzing novel ways to remove subcutaneous implants — which are applied under the skin — he never imagined it would lead to the product he currently has in development.
“It started off as a senior design project at Georgia Tech,” Mathew said. “I was getting a biomedical engineering degree. I was fairly new to the space and had no idea that implantable and drug delivery was as big as it is.”
The research engrossed Mathew, and he continued it after college. As he started interacting with patients and physicians, he realized there wasn’t just a need for new ways to remove implants, there was a need for whole new products.
Now, Mathew is the CEO of Hera Health Solutions, a pharmaceutical device company based in Memphis that specializes in bioerodible drug delivery implants. The startup moved from Atlanta to Memphis when it participated in the Memphis Bioworks Foundation's ZeroTo510 program, an accelerator for medical device innovation.
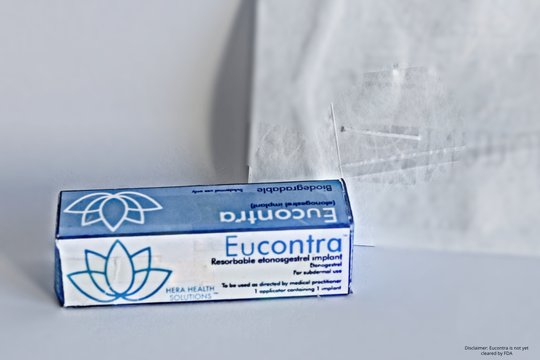
Hera's first product is Eucontra, a biodegradable contraceptive arm implant.
“What’s really opened my eyes,” Mathew said, “is that billions of dollars in the past decade have been poured into research that surrounds the creation of new medications and novel drugs. However, oftentimes, the delivery mechanism of these medications are still stuck in the 1950s.”
Medical implants are popular because they’re easy to put in the body, and they often can stay in place for more than a year. Many are delivered subcutaneously underneath the arm. Those implants are typically made out of ethanol vinyl acetate or silicone, however, which means they have to eventually be removed.
And this is where problems typically arise.
“Issues surrounding these removal processes have been growing because it’s difficult to remove the implant,” Mathew said. “It’s essentially a matchstick size, a tiny little rod that’s underneath the arm.”
Since the implants are so small, and because they’ve typically been in the body for a year or more, just finding them can be a challenge. And they’re removed with traditional scissors and forceps, Mathew says, which can lead to complications that include heavy bruising, scarring, and, at times, migration of the implant.
“I personally had the opportunity to witness over a hundred of these removal applications,” Mathew said. “I saw many different physicians, OB-GYNs, and nurse practitioners, experiencing the exact same issues over and over again.”
According to Mathew, more than a thousand cases regarding complications of implant removal procedure were reported to the U.S. Food & Drug Administration in 2018.
“It’s a side effect that can and should be alleviated,” Mathew said. “I think it’s a plus to both the OB-GYN and nurse practitioner who are doing these removals, and to the user who doesn’t have to deal with an invasive procedure.”
Because Eucontra is bioerodible, the need for a removal procedure is eliminated.
The product — which is under development and recently concluded its first round of bench and validation trials — is targeted to be a 12-to-18 month implantable.
Already, it’s received attention from around the world.
“We’re really excited about some of the NGO [nongovernmental organization] and nonprofit partnerships that are really giving us a warm welcome with the product, and the potential global impact it can have,” Mathew said.
In September, Hera Health was one of 30 companies invited by the United Nations to the Citypreneurs conference in Seoul, South Korea, where it was able to look for additional partnership opportunities and showcase its product. The U.N. invited companies that were advancing its sustainable development goals.
The company has also just closed a $1.25 million series seed financing round that was led by Innova Memphis, a local venture capital firm that focuses on biotech. There were others involved in the deal as well, including several health care investors based out of Ireland.
The money will allow Hera Heath to explore new possibilities, in addition to Eucontra.
“We’ve already received interest in many different applications, notably in the veterinary space and opioid addiction treatment,” Mathew said. “With this new financing, we are going to be allotting some of that capital to additional research and development, to investigate and hopefully launch some new products within the pipeline.”
The primary focus right now is its flagship product, which Mathew hopes will be approved by the FDA and the WHO (World Health Organization for International Markets) by the fourth quarter of 2021.
And when Eucontra does hit the market, Mathew thinks the worldwide effects could be significant.
“In areas that have limited access to health care,” he said. “A product like Eucontra could be huge.”