A Houston medical device company landed a Series A round and hit a milestone in the Food and Drug Administration approval process.
VenoStent closed a $16 million funding round led by Charleston, South Carolina-based firms Growth Capital and IAG Capital Partners, according to a July 31 news release. The money will be used to fund the company’s pivotal trial, which is a clinical trial used to demonstrate safety and confirm any adverse effects.
Local investors in the round included the Texas Medical Center Venture Fund and Baylor University’s Baylor Angel Network. Others include Oakland, California-based Creative Ventures, Fort Worth-based Cowtown Angels, and Manchester, New Hampshire-based Alumni Ventures.
The funding round follows VenoStent securing Investigational Device Exemption status from the FDA in May 2023. The classification means VenoStent can collect clinical data for its SelfWrap medical device through a trial known as the SelfWrap-Assisted ArterioVenous Fistula Study or SAVE-FistulaS.
“On the heels of our very promising results in several preclinical studies and a 20-patient feasibility study that led to our [FDA] Breakthrough Designation last year, this recent IDE approval is perhaps our biggest milestone to date,” said Dr. Timothy Boire, who co-founded VenoStent and serves as president and CEO.
SelfWrap is targeted for patients of vascular surgery by providing mechanical support to surgical connections between patient arteries and veins. According to VenoStent’s announcement, 5 million vascular access surgeries are conducted annually.
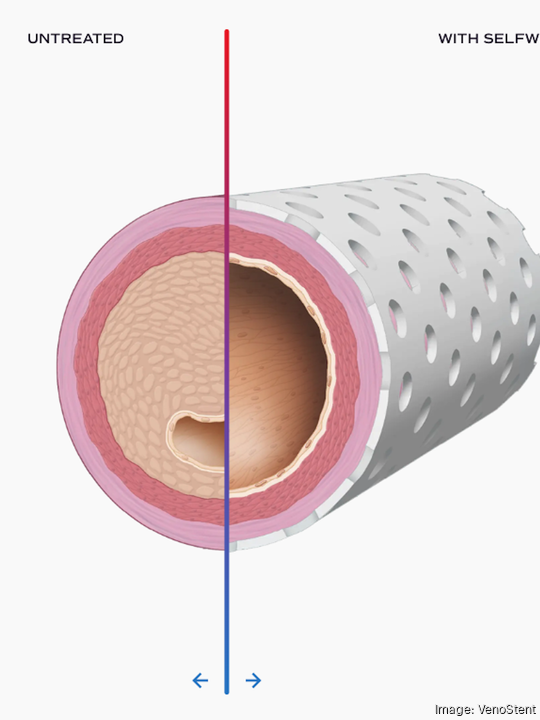
The company has previously received funding from federal agencies such as the National Science Foundation and the National Institute of Health as well as major national startup investors such as Y Combinator. According to its Crunchbase profile, VenoStent has raised $22.2 million to date.
The company was accepted into the Johnson & Johnson JLabs cohort at the TMC in 2018 and has an office presence there. VenoStent was originally spun out of research from Vanderbilt University in Nashville.
After landing at JLabs, VenoStent was accepted into the TMCx accelerator program, developed from the TMC Innovation Institute. The company also won at the 2018 Texas A&M New Ventures Competition.
Recent local life sciences investment saw the Cancer Focus Fund, affiliated with the University of Texas M.D. Anderson Cancer Center, invest $4.5 million into Houston-based ImmunoGenesis for the company’s own clinical trials in June. ImmunoGenesis confirmed the funding is part of a larger round that would be completed later in 2023.
Meanwhile, a potential investor for Houston’s life sciences scene moved into the Texas Medical Center Helix Park, which will open its first phase this year. Chicago-based Portal Innovations will operate 30,000 square feet of office and lab space in the TMC3 Collaborative Building on the 37-acre campus, and CEO John Flavin told Houston Inno that he hoped Portal would draw in capital from other parts of the country.
The TMC Venture Fund received a $50 million injection from its board of directors last year, which is intended to support investments for the next decade. Other TMC-backed companies include Houston-based health tech company Medical Informatics Corp., which landed a $17 million Series B round last year.