
An innovative syringe technology is one step closer to market. The company behind the device is gearing up for a Series A funding round.
True Concept Medical Technologies LLC (TCMT), a medical device innovation company in Dayton, has created two new syringe types designed to save lives and reduce cost of care while minimizing the opportunity for human error.
TCMT focuses on delivering devices designed by clinicians for clinicians in areas with significant morbidity and mortality such as sepsis and sudden cardiac arrest.
Cardiovascular disease kills 17 million people a year, making it the leading cause of mortality across the globe, according to TCMT. About half of those deaths are caused by heart attacks, but TCMT aims to provide a new tool for emergency health care providers.
TCMT Founder Mick Hopkins has over 20 years of experience as an emergency room nurse. Hopkins has invented a series of dual-syringe technologies to address needs he repeatedly encountered on the job.

Hopkins and his team are gearing up for a $15 million Series A funding raise to finish development and rapidly pursue regulatory clearance for its innovative technologies. The majority of which will go toward the company's D'VIA syringe.
D’VIA Syringe – Increasing accuracy for sepsis diagnoses
Of Hopkins' two devices, the first one expected to hit the market is D'VIA which aims to eliminate the $8.3 billion hospitals lose annually treating false-positive diagnoses for sepsis.
False-positives occur when the blood culture specimens – the gold standard to diagnose sepsis – are contaminated during collection.
There are an estimated 40 million blood cultures collected at hospitals around the country each year, TCMT says. Of that 40 million, 8% are positive for sepsis and 40% of those positive tests are false-positive.
This results in unnecessary prescription of antibiotics, increased hospital stays and additional financial burden to patients, providers and payers.
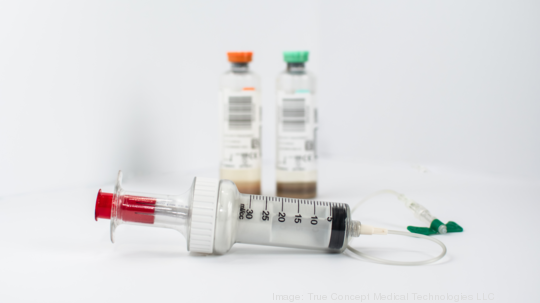
D’VIA will utilize a four-in-one dual-chamber design to isolate the first three milliliters of blood likely to contain contaminates. This section of blood will be repurposed for other testing rather than wasted.
The remaining chambers ensure a full 20-milliliter of clean blood is collected and dispenses the appropriate amount of blood into each blood culture bottle.
“I’m confident that the D’VIA dual chamber blood collection system has the potential to positively impact the care of septic and suspected sepsis patients across the entire population,” said Kettering Health Medical Director Dr. Michale Lakes.
SAFE Syringe – Treating cardiac arrest
Another technology TCMT is developing is called SAFE Syringe — geared toward treating cardiac arrest.
Certain cardiac conditions currently require medication followed rapidly by a 20-milliliter normal saline flush so the medication reaches the heart immediately. The current setups to administer these medications can take longer than four to six minutes and are prone to error in administration with 90% of clinicians failing to flush enough saline through after the medication, according to a release from TCMT.
TCMT designed the SAFE Syringe with an advanced two-in-one dual chamber to enable pre-loaded medications followed by a 20-milliliter flush to be delivered in one step. This ensures the correct amounts of medication are delivered rapidly to the heart.
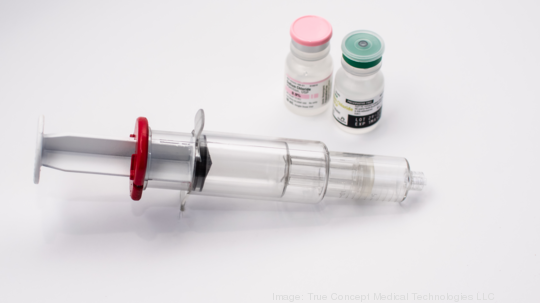
"I see this revolutionizing the way code medications are given," said Dr. Jordan Bonomo, director of critical care in emergency medicine, University of Cincinnati College of Medicine.
The SAFE Syringe is the first of Hopkins’ syringes to be prototyped over the last year with support from the MedTech Launch Fund.
The MedTech Launch Fund — managed by Launch Dayton partners Parallax Advanced Research and Entrepreneurs' Center — helps researchers bridge the gap between research and physical prototype to be shared with investors. The fund is financially supported by Ohio Third Frontier and the Economic Development Administration’s i6 program.
Hopkins was one of the first founders accepted into the MedTech Launch Fund portfolio.
"It's been a tremendous experience, to see your idea come all the way through to fruition and actually have a working prototype," Hopkins said. "It was a catalyst to take everything to the next level, build out the business, and really prepare us for an exciting 2022 and 2023."







