Nationwide Children's Hospital spinoff Andelyn Biosciences Inc. has completed its main corporate center and biotech manufacturing facility that eases an industrywide bottleneck for producing cell and gene therapies.
The 180,000-square-foot building off Lane Avenue at Carmack Road – the first private company to locate within the 270-acre Ohio State University Carmenton innovation district – hosted an outdoor celebration last week with balloons, face-painting, food trucks and a brass band playing Uptown Funk.
The massive high-tech facility signals new hope for families of children born with rare and often fatal conditions, said Andelyn CEO Wade Macedone.
"Working in gene and cell therapy, you get to know the patients' names, (and) their parents, going through a disease that is devastating for them," Macedone said. "It means the world to me. It means the world to the team. It means the world to the researcher.
"There are products we'll manufacture for one patient. ... This team does it in their free time – it's not just a job to them."

Macedone, promoted to the top job from COO in February, started with the business five years ago when it was a division inside the Columbus pediatric hospital.
Since spinning out in early 2020, Andelyn has grown to a staff of 250. About a dozen remain in labs inside the hospital's Wexner Research Institute, and in April 2022 opened a research center in Dublin, where about 30 employees complete toxicity studies and develop processes that then can be applied at the main site.
Pall Corp., which makes bioreactors and other equipment, invested $150 million in 2021 for a minority stake. Children's remains majority owner, according to the hospital audit.
While inside the hospital, the group had only the capacity to make batches large enough for the earliest research or the first safety trials in humans. Andelyn's new facility has the full scale for commercial production and packaging of approved therapies.
"We can maintain manufacturing in this facility all through the life cycle of a program," Macedone said.
The building was designed to "up the game" for preventing contamination in its clean rooms.
"With the enhanced capabilities this building provides, we will be better equipped than ever to advance the frontiers of biotechnology," said Dr. Dennis Durbin, president of the Wexner Research Institute at Children's.
Startups that spin out or established pharmaceutical companies that license tech from the hospital are likely to gravitate to the region to be near their clinical trials at the hospital, Durbin said. Now they also have plenty of local manufacturing capacity.
"We're really starting to shift the center of the universe for gene therapy here," Children's CEO Tim Robinson said.

Andelyn has about 165 clients ranging from university labs to pharmaceutical companies, Macedone said. New clients joined as the facility came online and some earlier clients that had left for larger manufacturers are returning.
Revenue was $21.4 million in 2022, according to Children's audit.
"We've now got a density of biotech here that's going to feed that innovation pipeline and attract talent from all over the country," said Eddie Pauline, CEO of the Ohio Life Sciences trade group. "If we can continue to create a 'sticky' environment in Columbus, we have the talent, the systems and the funding for these companies to grow."
The building, which exceeded $200 million, was completed on time and on budget despite inflation and labor shortages in recent years. Part of the manufacturing started ahead of schedule in fall because of pressing client needs.
Manufacturing capabilities went live roughly in the order they would be needed for developing a therapy. Commercial scale production equipment was commissioned in January.
The final section, expected to be validated to start operating this summer, is literally called "finishing," for repackaging the multi-gallon batches into labeled patient doses.

The combined experience of its staff is creating processes for more consistent and concentrated batches of the hard-to-grow biological material, Macedone said.
The manufacturer receives frozen test tubes of a client's desired genetic material, then grows those inside cells in ever larger batches. The genes are then inserted into modified viruses that can't cause disease or replicate themselves outside of the lab, but deliver genes to the body.
"It's going to change the industry, mature the expectations," Macedone said. "We can actually drive down cost ... and improve the quality of these products."
That could start to move the needle on the ultimate cost of the therapies, currently the most expensive drugs on the market, he said.

Andelyn had looked at other areas in Central Ohio for the greenfield development, but the innovation district gave it better access to OSU Wexner Medical Center and other advanced researchers. The Pelotonia Research Center, with several biotech interdisciplinary research "neighborhoods," opened in May.
"It's a fantastic location from a recruiting standpoint," Macedone said. "The energy is quite positive."
Central Ohio's growing biotech industry – including large startups such as Forge Biologics Inc. in Grove City and AmplifyBio LLC in West Jefferson – makes it easier to recruit doctoral-level candidates from the coasts, he said. They know if one job doesn't work out, there are other nearby companies hiring.
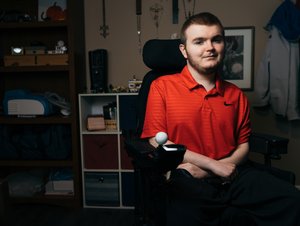
The 'And' in Andelyn visits the new biotech
Andrew Kilbarger, who represents the "And" in Andelyn, attended the grand opening with his parents and siblings. The 26-year-old from Lancaster has outlived most born with Duchenne muscular dystrophy through medical management of the condition at Children's.
In 2006, Kilbarger was the first human to test the safety of injecting a corrected segment of the Duchenne gene into a human body. At that time, development of gene therapies had stopped because of a death in 1999.
As an 8-year-old, the injections to his arms were just another in a long line of procedures to him.
"I never thought I'd be part of something like this," he said at last week's event. "Because of that, they made more therapies. (If I hadn't been the subject,) somebody else would have."
The same day as the opening, the U.S. Food and Drug Administration gave limited approval to the final version of the gene therapy for Duchenne. Children's licensed the tech to Sarepta Therapeutics Inc., based in the Boston area with a Columbus research center.
Evelyn Villareal, who makes up the rest of the company name, as a newborn was the first human treated with a Children's-invented gene therapy for spinal muscular atrophy, which received FDA approval in 2019. Her family could not attend the Andelyn opening.
"We share the name," Kilbarger said. "I've yet to meet her though. I would like to."