Just a few years after the first gene therapies were approved for the market, more than 1,000 new therapies are in clinical trials to determine whether they can beat rare and often fatal inherited disease.
Patients are waiting – and a Columbus biotech manufacturer started operating this month to ease the severe shortage in capacity to make the engineered genes and viruses that deliver them.
Furthermore, Andelyn Biosciences Inc. has the ability to turn around projects in three to five months – compared with an industry standard of six to 18 months, said Eric Blair, chief commercial officer.
"The days of genetic diseases are numbered," said Kenneth LaRiviere, Andelyn, who leads engineering. "Great things are going to be coming out of those doors for mankind, and that's just awesome."
The Nationwide Children's Hospital spinoff opened part of its manufacturing side to meet client demand while construction continues on the office portion at the other end of the 200,000-square-foot building off Lane Avenue. Andelyn is the first private company to locate within the 270-acre Ohio State University Innovation District, recently renamed Carmenton.
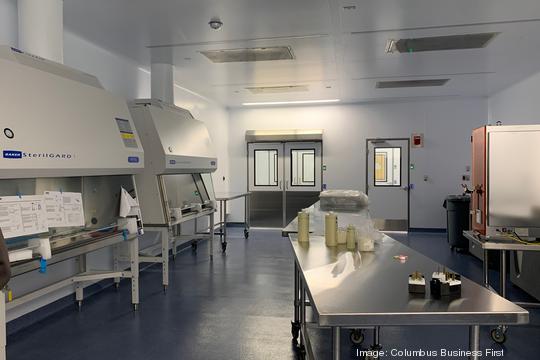
Check out the slideshow above for a tour of Andelyn's ultra-clean biotech manufacturing space.
After building out its in-house manufacturing capabilities, Children's spun out the for-profit company in January 2020 to help ease the industry's severe manufacturing bottleneck. Pall Corp., which makes bioreactors and other equipment, also made an undisclosed investment.
The startup had taken larger spaces inside the hospital's Wexner Research Institute, which now can open up for other projects. While inside the hospital, Andelyn had not been able to make batches larger than those for phase 2 clinical trials, which have a small number of patients.
The new facility can house 2,000-liter bioreactors – enough to purify and repackage batches into thousands of vials for a late-stage clinical trial, or for sale once approved.
Its eight identical manufacturing suites feature one-way traffic and individual air-handling units to prevent contamination. Crews roll in the needed equipment for unique client projects.
Andelyn started moving into the manufacturing side of the building in July, beating a target of the start of 2023. The office portion, about one-third of the building, should be complete by January. As client demand grows, there's room to expand the building westward.
The company managed to greatly speed up its construction schedule despite supply chain disruptions and intense competition for building trades labor in Central Ohio, LaRiviere said. The company and contractor Gilbane Building Co. accomplished the feat through careful orchestration of supplies and efficiencies in the build process.
The project was estimated at $200 million two years ago; the company is not releasing the final amount.
Meanwhile Andelyn seeks a new leader: CEO Mayo Pujols left in July for a publicly traded gene therapy development company, according to a news release and regulatory filings. Rocket Pharmaceuticals, Inc. (NASDAQ: RCKT) hired Pujols as chief technical officer and executive vice president.
Andelyn is not commenting on the CEO search, but Blair said Pujols remains in contact.
"We have a very strong leadership team that didn't miss a beat," LaRiviere said.
Nationwide Children's confirmed it still does business with its spinoff. Other clients are confidential, but include nonprofit foundations, universities, governmental agencies, biotech startups and pharmaceutical giants.
The list could grow to more than 100 by the end of 2023, now that Andelyn has the capacity for commercial-scale production, Blair said.
"It all starts with a frozen test tube," LaRiviere said.
Clients send frozen vials of their desired genetic material – Andelyn's receiving warehouse has freezers that can accommodate an 18-wheeler. The manufacturer then multiplies that by growing the genes inside cells in ever larger batches. The genes are then inserted into modified viruses that can't cause disease or replicate themselves outside of the lab, but deliver genes to the body.
Each batch takes weeks, perfectly calibrating the liquid growing media, temperature and gases that bubble through the mix, while testing the purity and volume. Andelyn's processes have produced yields triple the industry standard, LaRiviere said.
Andelyn also has opened its 55,000-square-foot research center in Dublin, where it completes toxicity studies and develops processes that then can be applied at the main site. Unlike a robot moving auto parts, the process is different every time to grow living things.
Some of the largest manufacturers in the industry are Thermo Fisher Scientific Inc. and Catalent Inc.
"We've now put ourselves in that class," Blair said. "The difference for us is we've been attached to patients for 20 years."
The startup's roots in a hospital bring profound experience that motivates speed and quality, he said. Employees transferred to Andelyn who treated the first children ever to receive a gene therapy, now on the market, that halts a fatal muscular condition.
"There's a very strong pioneering group here," Blair said.
LaRiviere, an engineer with an MBA, has 35 years of experience in facilities and process design in biotech, including 27 years leading vaccine production at pharmaceutical maker Merck. He joined Andelyn in June 2021.
"In all my years, this is the best (facility)," he said. "I love to design and build, so when this opportunity came up, I jumped at it."