A Nationwide Children's Hospital spinoff has received the FDA OK to test its novel method of fighting antibiotic-resistant infections in humans, starting the process toward seeking regulatory approval.
Clarametyx Biosciences Inc. announced Wednesday that the U.S. Food and Drug Administration accepted the Columbus company’s Investigational New Drug application, which means it can start a brief study evaluating the safety of the antibody treatment in healthy volunteers, followed by testing safety and gathering early effectiveness data in patients hospitalized with bacterial pneumonia.
"We’ve made tremendous progress with the early development of this potentially game-changing technology to specifically address bacterial biofilms, which is a major driver of resistance in bacterial infections,” CEO David Richards said in a news release. “We are now advancing as a clinical-stage company and can accelerate our efforts to identify the optimal application of this technology to enhance antibiotic approaches, with the potential for significant impact in treating severe bacterial infections and improving health outcomes.”
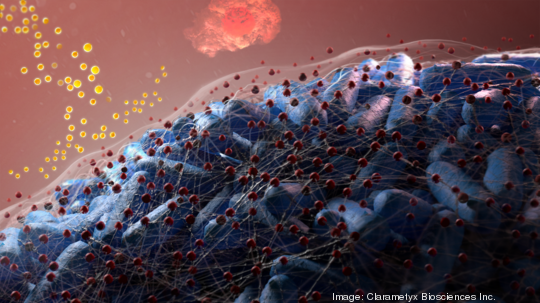
Inventors Lauren Bakaletz and Steven Goodman, Children's microbial researchers behind several of the Columbus hospital's patents, have for more than a decade targeted the slimy "biofilm" that encapsulates infection sites, protecting bacteria from the immune system and antibiotics. The antibody treatment attacks a key structural protein – universal regardless of the bacteria's species – so the film collapses. (The company has cool animations of this on its website.)
The biotech launched in early 2020 with backing from Ohio Innovation Fund and Rev1 Ventures. It also has attracted government and philanthropic research funding to develop the treatment without giving up equity. So far it has drawn $9 million from a $14 million award released in phases from a nonprofit research collaborative seeking treatments for antibiotic-resistant infections.
Biofilms are responsible for four out of five bacterial infections, according to the National Institutes of Health, including pneumonia and other respiratory infections, chronic sinus and ear infections and some resistant infections in wounds. Resisting treatment, these infections lead to more frequent and longer hospitalizations, and can be fatal.







