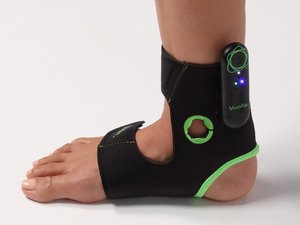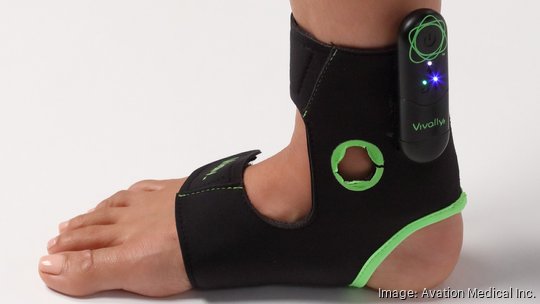
Jill Schiaparelli always carries an ankle wrap with an embedded electrode in her bag, just in case there's a chance for a conversation about bladder control.
"You never know,” said the co-founder and CEO of medical device startup Avation Medical Inc. “You just have to give people a reason to talk about it.”
Vivally System, the Columbus startup's FDA-approved wearable device and accompanying app, is now available by prescription in select markets including Ohio as a treatment for overactive bladder. Avation recently raised $22 million to fund the launch.
“I got to be where the first commercial patient was prescribed the product – and she cried,” Schiaparelli said. “I’ve met many of our patients. We had a teacher who was afraid of losing her job because … she was having to go to the bathroom every hour.”
OAB is frequent, uncontrollable urgency to urinate, sometimes causing incontinence. Conservative industry estimates are that it affects 1/6 of the population, Schiaparelli said, so people who ask her about the ankle wrap almost always mention a loved one with the condition. Existing treatments include drugs with side effects, weekly doctor visits for nerve stimulus with a needle, or surgical implants.
Avation's noninvasive device stimulates a nerve running up the back of the leg, which leads to a key relay point for nerves to the bladder. It's worn for about half an hour a few times a week.
“We started with a blank sheet of paper – with the patient in mind,” Schiaparelli said. “The way (care is) delivered today is nonsensical.
“I had a vision we're going to transform the way OAB is treated around the world, and we worked backward from there," she said.

How was Avation founded?
Renamed early on from Veressa, the startup is one of two formed by Ohio State University's former Neurotechnology Innovations Translator, which quietly shut down in 2019. The enterprise received $10 million of a $21 million Ohio Third Frontier award, according to the state Department of Development.
A serial entrepreneur, Schiaparelli started as a consultant for a market study on overactive bladder, then joined as CEO in 2017.
Schiaparelli worked several years at Johnson & Johnson, leading market launches for disruptive medical devices, then left for startups. This is the fifth medical device company in which she's served on the executive team.
Avation had to overcome doubts as she and engineers brought the design through several iterations, she said. The company owns all of its intellectual property, and now has 18 employees.
The end result not only had to be effective for patients, but appeal to the urologists who would prescribe it and insurers that would pay for it.
“Why do so many med-tech companies fail? They don’t deliver value for all three of those key stakeholders,” Schiaparelli said. “We were trying to anticipate the problems before they happen.”
Why invent a new treatment for overactive bladder?
Overactive bladder is chronic and incurable. Drugs to minimize urgency include anticholinergics, which are not recommended for patients older than 50 because of an association with dementia, and beta-3 agonists, which come with their own list of side effects including urinary infections and high blood pressure.
“It doesn’t make sense to me that people are being forced to put drugs in their body with all the side effects,” Schiaparelli said. “It’s not for a couple of days – you’re on this forever.”
Patients are required to try multiple drugs and fail to get relief before being considered for surgical implants. An device introduced in 1997 requires implanting electrodes near the spine in the hips, and new operations when batteries wear out after a few years. A newer device has a battery with a wireless recharger.
Other implants are for the tibial nerve by the ankle, but still require surgery.
“Patients don’t want surgery for what they consider a quality-of-life condition,” Schiaparelli said. “They need something that fits more easily to their lifestyle.”
To Schiaparelli the obvious answer was a wearable device. People kept telling her it couldn't work.
“You have to make it as easy to use as the click of a pen,” Schiaparelli said. “That’s where we spent a lot of time in innovation.”
What did clinical studies show about Vivally?
"Significant" reductions in urgency episodes, trips to the bathroom and incontinence were reported in a study of nearly 100 patients, published online by the journal Urology in November. Subjects divided in groups were instructed to use the device either once or three times weekly for 12 weeks, then every other week for a year.
"Long-term results remained robust at 12 months," the abstract said.
The journal put the study on the cover of its January print issue, which goes to physicians across the country.
“That’s sort of a marketing gift,” Schiaparelli said. “We never in a million years imagined they would put a picture of the product on the cover.”
The stimulator is built in northern Ohio and final product assembled and shipped from Columbus. Avation aims to develop more devices.
“We want to make neuromodulation accessible to a wide variety of people across a wide variety of conditions,” Schiaparelli said. “The way you do that is a wearable product.”
The company has raised earlier funding rounds while in stealth mode, but does not disclose cumulative funding raised.
“The hard part starts now,” Schiaparelli said. “We have a commitment to our investors, our patients, our physicians – we want to get it right.”