A Cincinnati medtech startup is celebrating a “watershed moment” as it edges closer to commercialization following a key approval from the U.S. Food and Drug Administration.
Sense Neuro Diagnostics said the FDA approved its application to move forward with a new clinical trial to develop a device for detecting bleeding in the brain – a study that will stand as its final hurdle before market launch.
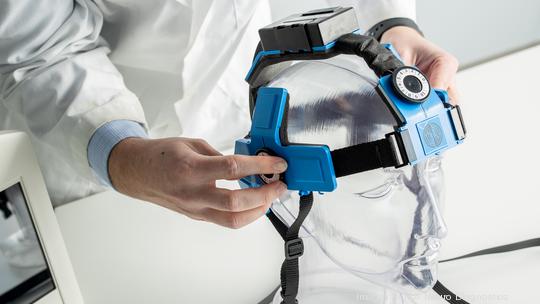
Norwood-based Sense Neuro, founded in 2014 by four University of Cincinnati physicians, is developing multiple noninvasive brain scanners. Its noninvasive technology has the potential to collect 360 data points in 2.5 seconds to detect brain hemorrhage or stroke type, helping first responders, emergency department personnel, neuro ICU teams and military field hospitals assess and monitor traumatic brain injuries.

The new trial – approved by the FDA Division of Neurosurgical, Neurointerventional and Neurodiagnostic Devices – will begin as soon as June and involve up to 300 patients at 30 sites in the United States, Canada and India. It will focus on validating the company's algorithm for hemorrhage detection.
More than 20 sites across the U.S., Canada and India have already engaged with the startup for a prior pivotal trial evaluating an in-hospital device, and five U.S. Department of Defense sites have been evaluating the company’s military field device.
Sense Neuro executives hope to complete the new study by the end of the year – at which time they will submit to the FDA for clearance.
“Once we finish this last clinical trial, we hope to be in a position to celebrate Sense’s first commercial device and its launch into the marketplace,” Geoff Klass, CEO of Sense Neuro Diagnostics, said in a news release.
“This trial should be our last hurdle before we establish a commercial platform and transcend beyond startup status to a commercial company. It’s a watershed moment for our entire team and everyone who has supported Sense in its nearly 10 years of effort.”
Sense, which calls Norwood-based Alloy Development Co. home, has been aiming for a 2024 commercial launch, given its trial progress and a recent “know-how” license agreement it inked with the Mayo Clinic to advance its military device.
The startup has developed its technology in collaboration with the University of Cincinnati Medical Center. UC Medical Center and Grady Memorial Hospital at Emory University in Atlanta are committed to participate in the next clinical trial, and Sense Neuro said it intends to partner with research sites that have participated in its previous trials.
To date, the company has raised more than $14 million in total funding, including equity from investors including Queen City Angels, Accelerant Ventures in Dayton, Cleveland Clinic, the Global Cardiovascular Innovation Fund and a private group of Cincinnati-based neuro physicians.
Dr. Opeolu Adeoye, chief medical officer at Sense Neuro Diagnostics, called the technology "a springboard" for a series of product launches the team anticipates over the next several years.







