A Greater Cincinnati medtech startup has received a key designation from the U.S. Food and Drug Administration that could help fast track the widespread availability of its product for millions of patients with potential heart problems.
Genetesis, a 7-year-old Mason-based medical device company that counts famed investor Mark Cuban as one of its earliest backers, has been granted “breakthrough device designation” by the FDA for its flagship technology CardioFlux. The news comes on the heels of a more than $10 million raise earlier this year.
Genetesis initially applied for the breakthrough device designation in October; the goal of the program, the FDA said, is to speed up product development, assessment and review.
CEO and co-founder Peeyush Shrivastava told me the designation will serve as a “catalyst” in making CardioFlux available for widespread clinical use, something he said should happen in 2021.
“It’s a testament to the FDA believing in the potential of this technology,” he said. “A lot of value comes with that. We get regular FDA interaction, ultimately for clinical approval of our technology, which would make it widely available throughout the United States. It also creates opportunities for early reimbursement, which would make it financially meaningful for hospitals and other providers to adopt early next year.”
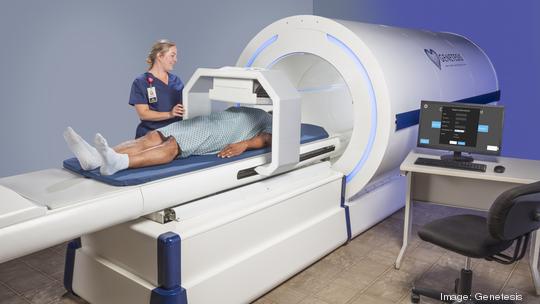
The FDA designation is also further proof, Shrivastava said, that CardioFlux meets an important, unmet clinical need. CardioFlux offers an alternative to traditional cardiac stress testing, which often involves exercise, the use of pharmaceuticals and/or radiation exposure. CardioFlux, which is a non-invasive biomagnetic imaging system, can measure and visualize the electromagnetic function of the heart in a 90-second scan.
Undiagnosed chest pain sends 8-10 million Americans to emergency departments every year, and 75% of those cases are not heart-related. Despite this, assessing chest pain remains inefficient and expensive, Genetesis said. Often the triage process can take 8-24 hours.
Genetesis said CardioFlux represents a next-gen evolution. Genetesis, a Cincy Inno “Coolest Company,” received its first FDA 510(k) clearance in 2019.
Next steps include a roughly 500 patient, multi-center clinical trial, which will include at least one unnamed site in Ohio. Through the breakthrough device program, Genetesis will be reviewing what’s called a data development plan with the FDA. After the trial, the company can submit for FDA clinical approval.
“That will be the landmark,” Shrivastava said. “That’s when the floodgates open."
Genetesis, founded in 2013, is part of a growing bio hub in the city of Mason, which also includes companies like IncludeHealth, Clarigent Health, Masters Pharmaceutical and the rebranded Myriad Neuroscience, formerly Assurex Health. The startup is located in the city’s Tech Elevator, which helps early-stage tech and bioscience companies scale.
Genetesis closed a $10.5 million Series B in April, led by TDK Ventures, a California-based corporate VC fund, and an undisclosed Fortune 500 firm, with participation from partners like CincyTech, the Ohio Innovation Fund and Cuban's Radical Investments. That brings its total funding to $20 million to date. By year’s end, Genetesis will employ 27, Shrivastava said.
"The FDA designation recognizes the positive patient impact that is possible with Genetesis,” CincyTech CEO Mike Venerable said. “This accomplishment is a credit to Peeyush and his team."







