Imagine this: Your bladder no longer works properly. Your doctor tells you scientists will grow a new bladder in a laboratory and then implant it.
That’s what researchers and scientists, led by Dr. Anthony Atala, at the Wake Forest Institute of Regenerative Medicine have been working on for more than two decades.
“When we started doing this research, people really thought it was science fiction,” Atala said. “They really did. They really thought there’s absolutely no way that you can grow tissue and put it into a patient.”
But there is a way to grow replacement tissue and organs: It starts by taking a few of a patient’s healthy cells from the affected tissue or organ and growing them in a laboratory. Once there are sufficient cells — often enough to cover an entire football field — scientists can populate an organ- or tissue-shaped mold, called a scaffold, with the new cells. The new organ or tissue can be implanted after it goes through a bioreactor to ensure the cells stay put. With this technology, there is no danger of the patient’s body rejecting the new organ because it was grown with his or her own cells.

Researchers at WFIRM were the first to show how this process of regenerative medicine works; in 1999, they successfully implanted lab-grown bladder tissue into a patient.
WFIRM is widely recognized as an international leader in the regenerative medicine field and has worked to develop many firsts. The institute’s researchers work on more than 40 different tissues and organs in four categories — flat structures, tubular tissues, hollow organs and solid structures.
Regenerative medicine has large market potential, particularly with lab-grown organs. There are currently 106,233 people waiting for an organ transplant, with another person added every nine minutes, according to the U.S. Department of Health and Human Services' Organ Procurement and Transplantation Network. Some 17 people die each day waiting for an organ transplant — a number that could decrease with the help of regenerative medicine.
The institute’s researchers have 15 applications of their cell- and tissue-therapy technologies, such as skin, urethras, cartilage and bladders, in patients, and they are working toward their 16th.
Outside of tissue and organ engineering, WFIRM researchers work on other therapies and technologies within regenerative medicine. For example, WFIRM scientists work on cell therapies that use living cells to promote healing and regeneration from within.
Another big area is bioprinting, or 3D printing organs. Once WFIRM’s scientists successfully created a lab-grown organ, they quickly realized they needed to scale up the manufacturing process for it to become commonplace.
The team of researchers took a desktop inkjet printer and modified it to print tissues. They essentially loaded it with cells instead of ink. Over 14 years, the researchers created the integrated tissue- and organ-printing system that deposits biodegradable, plastic-like materials to form the tissue or organ shape and water-based gels that contain the cells. In 2016, the researchers published a report proving the feasibility of printing living tissue structures.
Another project is the diagnostic-focused "body on a chip," in which scientists miniaturize tissues and organs and place them on microchips. With the help of microfluidic devices, scientists can create a system that represents the human body. Drugs and cancer chemotherapies are tested on the pinhead-sized, 3D versions of the tissues and organs.
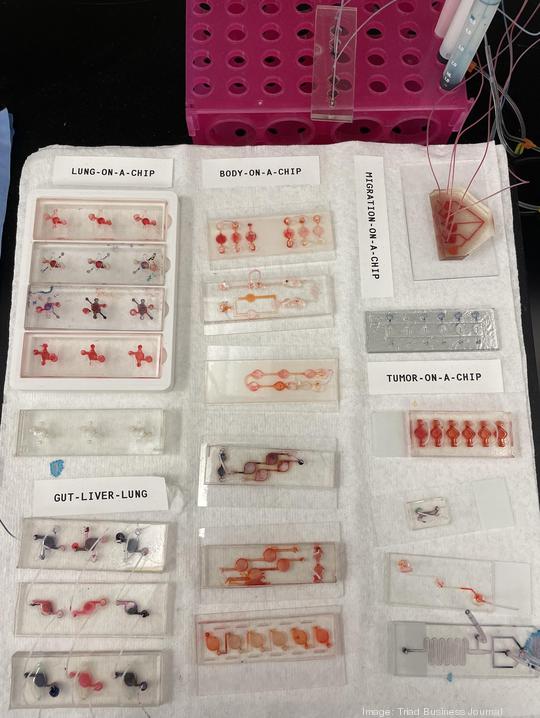
Atala notes 90% of drugs that enter clinical trials end up failing, saying a huge reason is because those trials use a population of cells or animal bodies that do not represent the human body. In the body-on-a-chip project, the miniaturized tissues and organs react as they would in a human body, allowing researchers to know whether a drug or therapy is effective.
WFIRM’s goal is to improve the lives of patients through regenerative medicine’s technologies and therapies — and it takes a wide-range approach to that goal.
“When you do that, the science has to be very, very solid,” Atala said. “You’re not doing that just for an experiment. You’re not doing that just to publish a paper. You’re basing that work on a solid foundation that has to be reproducible, done every single time the same way, so you can get it to a patient.”