Indica Labs, a medical technology company based in Albuquerque, said May 8 it received clearance from the Food and Drug Administration to take one of its flagship pathology products into clinical markets in the U.S.
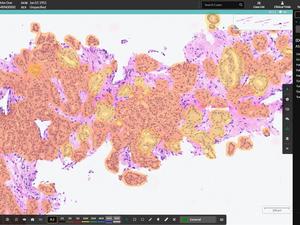
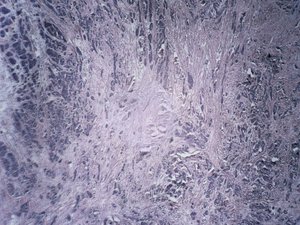
That product, called HALO AP Dx, is designed to assist pathologists in diagnosing disease via review and interpretation of digital images of scanned pathology slides — a digital, remote diagnosis process instead of relying on glass slides and optical microscopes only. It's built on the Albuquerque company's HALO AP software platform, which integrates artificial intelligence to make those diagnostic processes more efficient, according to a May 8 release from Indica Labs.
The 510(k) clearance from the Food and Drug Administration (FDA) means the Albuquerque company can now sell its HALO AP Dx product to general hospital or medical clinical pathology providers. This is the first FDA clearance Indica Labs has received for one of its pathology products, said Eric Runde, the company's chief operating officer, for use with a specific slide scanner for in vitro diagnosis.
"It gets us on to a whole new stage," Runde said.
That includes allowing other company products to be deployed and tested. Runde said Indica Labs plans to deploy various AI algorithms the company is working on, including artificial intelligence tools for prostate cancer and breast cancer diagnosis, through its HALO AP platform, leading to potentially more FDA clearances for those AI-backed platforms down the road.
Receiving this inaugural FDA clearance is also a boon for expanding Indica Labs' customer reach. The company previously received a clearance to use the platform for in vitro diagnosis in the United Kingdom.
"Other pharmaceutical companies or drug developers that we currently work with, knowing that we can build something and get it cleared by the FDA also lends a lot of credibility to what we're trying to do," Runde said.
Indica Labs currently deploys its software products with large pharmaceutical companies worldwide and reference laboratories like NeoGenomics Inc., based in Fort Myers, Florida. It also has an enterprise software license with the National Cancer Institute, Runde said.
Before the FDA clearance, Indica's HALO AP Dx product could only be used for research and testing purposes. Laboratories, Runde said, could previously "self-validate" the product in their labs, but the FDA clearance gives the product a "stamp of approval" that means the labs no longer have to "undertake that validation themselves."
The Covid-19 pandemic spurred a big push for "going digital" in pathology labs, Runde said.
"Now different labs are going digital, so they're looking for people who have FDA clearances and devices that can drop right into their lab," Runde said. "That's the big market opportunity for us, is all of these big clinical labs around the country or small clinical labs that are doing diagnoses day in, day out."
The clinical market, he said, is "much larger" than the life science research and testing market Indica Labs was previously able to access.
Indica Labs was founded in 2011 and in 2019 moved into a 14,500-square-foot building at 8700 Education Place NW. It currently employs around 115 people at offices in Albuquerque, the United Kingdom, Japan and China.
"Our team is thrilled to achieve this FDA clearance for HALO AP Dx," Steven Hashagen, Indica Labs' founder and CEO, said in a statement.
The majority of those employees are located in New Mexico, COO Runde said, and he added the company currently has openings for software engineers and AI developers.