A disease-detecting device under development at VisionQuest Biomedical Inc. is on the fast track for commercialization thanks to a $1 million federal grant.
VisionQuest's CEO, Peter Soliz, Ph.D., is developing a commercial prototype of a biomedical device called i-RxTherm. The device, once FDA-approved, would be used to detect a degenerative nerve damage condition in people with diabetes — called diabetic peripheral neuropathy — in its early stages.
The National Institute of Diabetes and Digestive and Kidney Diseases — part of the National Institutes of Health (NIH) — awarded Albuquerque-based VisionQuest the grant as part of its Commercialization Readiness Pilot Program. In addition to putting together the commercial prototype, VisionQuest will also use the grant money to gather data for FDA approval.
VisionQuest plans to have the commercial prototype ready in six months and apply for FDA approval within 12 months, said Sarah Soliz, director of strategic communications for VisionQuest. The NIH grant money will last about a year, Sarah Soliz said.
The i-RxTherm device records infrared videos of patients' blood flow to see if they may have diabetic peripheral neuropathy. The device is a "diagnostic aid" that would provide "quantitative data" to physicians while "producing little to no discomfort for the patient," Peter Soliz said in a press release.
Securing FDA approval would mark the first step toward commercialization for the i-RxTherm device, Sarah Soliz said. She said that getting approval would be "huge" for VisionQuest's profile and help the 10-person company secure additional funding, whether through more grants or private investors.
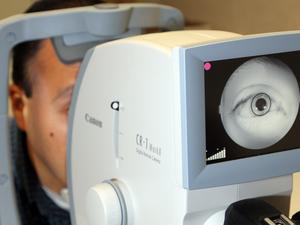
Peter Soliz founded VisionQuest Biomedical in 2007 with goal of simplifying the early diagnosis of diseases using artificial intelligence technology. EyeStar, its first software application, is used to diagnose diabetic retinopathy in patients' eyes, and its Aspire software detects malarial retinopathy. The new i-RxTherm marks VisionQuest's first technological effort into early-stage diabetic disease detection in other parts of the body besides the eyes.
The company got FDA approval in December 2020 for its software technology. Its Aspire tech won a nearly $1 million award in May 2021. It received a three-year, $3 million FDA grant, alongside the University of New Mexico, in June 2020.